Peptide-based Drug Research Group
In a near future, infections caused by multidrug-resistant pathogenic bacteria are likely to become a major threat to public health. Cancer is a leading cause of death with high costs for medical treatment. Discovery of therapies that effectively combat these diseases is of high societal value.
Chemotherapeutics comprise both antibiotics and anticancer drugs and common features are that their mode of action involves selective killing of cells (either microbial or cancerous) and severe problems with development of resistance mechanisms - either via bacterial selection processes or during cancer treatment of individual patients. To circumvent these issues, we design peptides and peptidomimetics capable of killing multidrug-resistant (MDR) bacteria as well as develop peptide-drug conjugates targeting cancer, MDR strains and circumvent drug-induced over-expression of efflux pumps.
- Identification of peptide-based antibacterial lead compounds
- Proof of concept for novel designs of antibacterial peptidomimetics
- Improved methods for on-resin peptide modification
Collaborators - National
Prof. Claus E. Moser, Dept. Clinical Microbiology, Rigshospitalet (Copenhagen University Hospital)
Prof. Anders Løbner-Olesen, Dept. Biology, University of Copenhagen
Prof. Peter E. Nielsen, Dept. Cellular & Molecular Biology, University of Copenhagen
Prof. Hanne Ingmer, Dept. Veterinary & Animal Sciences, University of Copenhagen
Prof. Luca Guardabassi, Dept. Veterinary & Animal Sciences, University of Copenhagen
Prof. Camilla Foged, Dept. Pharmacy, University of Copenhagen
Prof. Niels Frimodt-Møller, Dept. Clinical Microbiology, Rigshospitalet (Copenhagen University Hospital)
Prof. Gunnar Houen, Rigshospitalet, Glostrup
Assoc. Prof. Peter Thulstrup, Dept. Chemistry, Science, University of Copenhagen
Assoc. Prof. Majid Sheykhzade, University of Copenhagen
Assoc. Prof. Peter Damborg, Dept. Veterinary & Animal Sciences, University of Copenhagen
Statens Serum Institut (several senior scientists), Denmark
Collaborators - International
Prof. Magnar Bjørås, Dept. Microbiology, Oslo University Hospital, Norway & Dept. Cancer Research & Molecular Medicine, Norwegian University of Science and Technology, Trondheim, Norway.
Prof. Fernando Rogério Pavan, Sao Paulo State University, Sao Paolo, Brazil.
Assoc. Prof. Liam Good, Pathobiology & Population Science, Royal Veterinary College, London, U.K.
Assoc. Prof. Lorenzo Stella, Dept. Chemical Science and Technologies, University of Rome Tor Vergata, Rome, Italy.
Prof. Maria Luisa Mangoni, Dept. Biochemical Sciences, Sapienza University of Rome, Rome, Italy.
Prof. Paolo Rovero, University of Florence, Italy.
Prof. Johan Svensson, Cawthron Institute, New Zealand.
Dr. Dorota Zabicka, National Microbiology Institute, Warzaw, Poland.
Director, O. Pugovics, Latvian Institute of Organic Synthesis, Riga, Latvia.
Dr. Ilona Domraceva, Latvian Institute of Organic Synthesis, Riga, Latvia.
Prof. Raivis Zalubovskis, Latvian Institute of Organic Synthesis, Riga, Latvia.
Prof. R. E. W. Hancock, University of British Columbia, Vancouver, Canada.
Assoc. Prof. Annelise Barron, Stanford Bioengineering, CA, USA.
Assoc. Prof. Reidar Lund, University of Oslo.
Dr. Jeff Watts, Zoetis, Kalamazoo, Michigan, USA .
Prof. Claes Dahlgren, Sahlgrenska Academy, University of Gothenburg, Sweden.
Assoc. Prof. Ian Mellor, School of Life Sciences, University of Nottingham, U.K.
Assoc. Prof. Izuddin Fahmy Abu, Institute of Medical Science Technology, Universiti Kuala Lumpur, Kuala Lumpur, Malaysia.
Henrik Franzyk:
Norwegian Research Council (Collaborative and Knowledge-building Project Collaborative Project; 2022-2024): A platform for development of peptide-based antimicrobials for treatment of infections with drug-resistant bacteria. Collaborator share: 4.04 mio NOK (1 PhD and part-time lab technicians).
Danish Technical Research Council (PhD project; 2022-2024): Exploration of efflux pump inhibitor conjugates via Trojan-horse and shuttle approaches. Grant: 2.88 mio DKK.
Novo Nordisk Foundation Challenge project (Center for Peptide-Based Antibiotics: CEPAN; 2017-2022); partner share: 14.9 mio DKK.
Partner in ENABLE (IMI: “New Drugs for Bad Bugs 4”; 2014-2021); share: 2.5 mio DKK.
Paul R. Hansen:
Partner in FP7-PEOPLE-2011-ITN TRAIN-ASAP, (2012-2017)
Partner in Antimicrobial peptides seen by neutrons (2016-2020) funded by Nordforsk.
Master students
If you are interested in carrying out your MSc project (specialeprojekt) in the group, please contact henrik.franzyk@sund.ku.dk or prh@sund.ku.dk for a discussion of available projects within solid-phase synthesis, peptide chemistry of modification of drugs (e.g., antibiotics). Please provide a description of prior experience within practical organic and/or solid-phase synthesis together with a transcript of grades. Project duration is minimum 6 months (i.e., 30 ECTS), and preferably 12-months (i.e., 60 ECTS). International exchange students (e.g., with an ERASMUS grant) are welcome join, provided funding for travel and accomodation is already in place. Student research results typically become published in peer-reviewed journals when the overall research project is finalized.
PhD students
We do not currently have fully funded PhD fellowships available. Students with an EU scholarship (e.g. Marie Curie) or national grant are welcome to contact henrik.franzyk@sund.ku.dk or prh@sund.ku.dk.
When available, fully funded PhD scholarships will be posted on https://employment.ku.dk/phd/. Please apply only the online system, since e-mailed applications cannot be considered.
Also, visiting PhD students are welcome to contact henrik.franzyk@sund.ku.dk or prh@sund.ku.dk for a discussion of possibilities for a project within the group.
Postdoctoral fellows
We do not currently have fully funded postdoctoral fellowships available. Postdoctoral candidates with own fellowship are welcome to send their CV and letters of recommendation to henrik.franzyk@sund.ku.dk or prh@sund.ku.dk.
Postdoctoral fellows/Assistant Professors
Nicki Frederiksen (2020-2022; supervisor: H. Franzyk )
Ashif Yasin Shaik (2018- 2022; supervisor: H. Franzyk)
Anita Wester (2017-2020; supervisor: H. Franzyk)
Anna Mette Hansen (2014-2018; supervisor: H. Franzyk)
Kolja M. Knapp (2009-2011; supervisor: H. Franzyk)
Francois Crestey (2007-2009; supervisor: H. Franzyk)
Graduate students (= PhD students -2023 evt. incl. nuværende affiliation)
Nicki Frederiksen (2017-2020; main supervisor: H. Franzyk)
Malene Vinther Christensen (2015-2018; main supervisor: H. Franzyk)
Bala Krishna Prabhala (2014-2018; co-supervisor: P.R. Hansen)
Natalia Molchanova (2013-2016; main supervisor: H. Franzyk)
Alberto Oddo (2012-2015; main supervisor: P.R. Hansen)
Ines Greco (2012-2016; main supervisor: P.R. Hansen)
Sarah L. Skovbakke (2012-2016; main supervisor: H. Franzyk)
Gitte Bonke Seigan (2011-2014; main supervisor: H. Franzyk)
Christina Schjøt-Eskesen (2011-2015; co-supervisor: P.R. Hansen)
Rasmus D. Jahnsen (2010-2013; main supervisor: H. Franzyk)
Jens Kristian Munk (2010-2013; supervisor: P.R. Hansen)
Nicole Hartwig Trier (2009-2013; co-supervisor: P.R. Hansen)
Lars K. Ottesen (2007-2010; main supervisor: H. Franzyk)
Charlotte Lund Denholdt (2004-2008; co-supervisor: P.R. Hansen)
Dan Ifrah (2002-2006; co-supervisor: P.R. Hansen)
Trine S. Ryge (2001-2006; supervisor: P.R. Hansen)
Christian A. Olsen (2001-2004; main supervisor: H. Franzyk)
Malene R. Jørgensen (2001-2004; main supervisor: H. Franzyk)
Jacob A. D. Clausen (2009-2012; supervisor: H. Franzyk)
Jon H. Rasmussen (1995-1998; supervisor: H. Franzyk)
Signe M. Frederiksen (1995-1998; supervisor: H. Franzyk)
MSc, BSc, and visiting students
Julie Jin (2022-2023; PRH)
Lavan Sharif (2022-2023; PRH)
Murat Kalayci (2022-2023; PRH)
Serpil Ahmed (2022; PRH)
Anna Kristoffersen Foltmar (2021-2022; HF)
Abdullah Al-Awkati (2021-2022; PRH)
Nina Cecilie Lyck (2021-2022; HF)
Luise Pallasdies (2021-2022; HF)
Yunhao Duan (2021-2022; PRH)
Oguzhan Saglam (2021-2022; PRH)
Teodor Olejko (2021-2022; PRH)
Mohammad Firoznezhad (2021; PRH)
Ilaria Fanelli (2021; PRH)
Malak A. Suleiman (2020-2021; HF)
Farah Mughal (2020-2021; PRH)
Sannie Rafique (2020-2021; PRH)
Thomas Bobak (2020; PRH)
Stavroula Louka (2019-2020; HF)
Sahar Hashim Ali Hussein (2019-2020; HF)
Sarah Benali (2019-2020; PRH)
Azra Kapicic (2019-2020; PRH)
Maria Prokou (2019-2020; PRH)
Mahdieh Dagina Pedersen (2019; PRH)
Chirag Mudaliar (2018-2019; HF)
Rajan Ali (2018-2019; PRH)
Jasmina Rashid (2018-2019; PRH)
Nikoline Nielsen (2018-2019; PRH)
Georgia Karathanasi (2018; PRH)
Lars Sørensen (2017-2018; HFD)
Signe Jensen (2017-2018; PRH)
Malene Pedersen (2017-2018; PRH)
Ida Andersen (2017-2018; PRH)
Kathleen Mary Coyle: (2017-2018; PRH)
Jing Zhou (2017-2018; PRH)
Catarina Freire (2017; PRH)
Catarina R. Coelho (2017; PRH)
Niccolo Valdarnini (2017; PRH)
Sabbah Ahmed (2016-2017; PRH)
Wafaa Hamad Rissan Al-Mansour (2016-2017; PRH)
Christina Nielsen (2015-2016; HF)
Simon B. Christensen (2015-2016; HF)
Abdullah Lone (2015-2016; PRH)
KP Nandhini (2015; PRH)
Helen Mendel (2015; PRH)
Iris Perez-Gassol (2014-2015; HF)
Lena Münzker (2014-2015; PRH)
Johannes E. Hansen (2014-2015)
Marina Fuentes (2014; PRH)
Bardur Johannesen (2014; PRH)
Agnete Emborg (2013-2014; PRH)
Julie Harboe (2013-2014; PRH)
Ciara Gorey (2013; PRH)
Camilla J. Larsen (2012-2013; HF)
Paola Saporito (2012-2013; HF)
Maria Leth (2012; PRH)
Frederikke Fliedner (2012)
Paula Melo Paulon Hansen (2011-2012; PRH)
Simon Welner (2010-2011; PRH)
Surekha Dipak Amrutkar (2010-2011; PRH)
Tanja Poulsen (2009-2010; PRH)
Rasmus Jahnsen (2009-2010; HF)
Jesper Søborg Bahnsen (2009-2010; HF)
Christian Hougaard (2008-2009; HF)
Arham Isa (2007-2008; HF)
Angelo Bella (2007; HF)
Maria Buccolo (2007; HF)
Sandra Lerche Nielsen (2005-2006; PRH)
Karin Jørgensen (2005; PRH)
Susanne Ladefoged Nielsen (2003-2004; PRH)
Ole Weltz (2001-2002; PRH)
Charlotte Lund Hansen (2001-2002; PRH)
Petrine Wellendorph (2000-2001¸HF)
Research areas
Infectious Diseases
(CEPAN; co-PI: H. Franzyk)
In response to the critically increasing worldwide threat to human health posed by emergence of bacterial resistance to currently used antibiotics, we have established a discovery platform focused on peptide-based antibiotics.
Membrane-permeabilizing adjuvant antibiotics are investigated as a means for circumventing resistance to current antibiotics and to sensitize bacteria to antibiotics that they are inherently resistant to. Also, synergistic combinations representing a multimodal treatment regimen are examined as an approach for reducing risk of resistance development. In addition, moieties that enhance bacterial uptake of potential antibacterial compound are explored. Ongoing studies concern development of novel cyclic lipopeptide antibiotics (e.g., analogues of colistin) as well as optimization of peptidomimetics exerting bacterial killing and/or potentiating effects when combined with existing antibiotics.
Center for Peptide-Based Antibiotics (CEPAN) is funded by an NNF Challenge grant and headed by Prof. P.E. Nielsen (Dept. Cellular and Molecular Medicine, University of Copenhagen). See more at: http://cepan.ku.dk
Group members (in the Franzyk lab) comprise: Previous (Postdocs/PhD student): Anita Wester (Delivery moieties for antibacterial antisense PNA oligomers), Ashif Yasin Shaikh (PNA synthesis and antibiotic-peptide conjugates), and Nicki Frederiksen (Antimicrobial peptides and peptidomimetics); current (PhD student): Johan Storm Jørgensen (Novel cyclic lipopeptide antibiotics).
Selected publications:
Frederiksen, N.; Loukas, S.; Mudaliar, C.; Domraceva, I.; Kreichberga, A.; Pugovics, O.; Zabicka, D.; Tomczak, M.; Wygoda, W.; Björkling, F.; Franzyk, H. Int. J. Mol. Sci. 2021, 22, 7041 (17 pages). https://doi.org/10.3390/ijms22137041
Mood, E. H.; Goltermann, L.; Brolin, C.; Cavaco, L. M.; Yavari, N.; Frederiksen, N.; Nejad, A. J.; Franzyk, H.; Nielsen, P. E. ACS Infect. Dis. 2021, 7, 2152-2163. https://doi.org/10.1021/acsinfecdis.1c00147
Frederiksen, N; Hansen, P. R.; Zabicka, D.; Tomczak, M.; Urbas, M.; Domraceva, I.; Björkling, F.; Franzyk, H. ChemMedChem 2020, 15, 2544-2561. https://doi.org/10.1002/cmdc.202000526
Frederiksen, N; Hansen, P. R.; Björkling, F.; Franzyk, H. Molecules 2019, 24, 4429 (18 pages). https://doi.org/10.3390/molecules24244429
Contact: Henrik Franzyk; henrik.franzyk@sund.ku.dk
(co-PI: H. Franzyk)
A worldwide health crisis is emerging as Gram-negative bacteria are becoming multidrug-resistant (MDR), leaving them to be treated with last-resort antibiotics only. As the pharmaceutical industry’s discovery and development of novel antibiotics often are based on existing compound classes, resistance often develops rapidly. This troublesome situation calls for an effort by academia to perform alternative research within future antibiotics.
Focus in the present project is on design and synthesis of compounds based on modification of newly identified hit peptides arising from the toxin part of bacterial toxin-antitoxin systems. The aim is to improve bacterial uptake, while minimizing toxicity toward mammalian cells. Also, incorporation of unnatural residues is used as a means to increase stability towards enzymatic degradation in vivo. Emphasis will be on identification and development of lead compounds against Gram-negative human pathogens (e.g., E. coli, Klebsiella pneumoniae, Acinetobacter baumannii and Pseudomonas aeruginosa). The project is funded by the Norwegian Research Council and headed by Prof. Magnar Bjørås.
PhD student: Emma Dyhr Jensen (Project title: Exploration of peptides derived from the bacterial toxin-antitoxin system as potential antibiotics)
Publication:
Sæbø, I. P.; Bjørås, M.; Franzyk, H.; Helgesen, E.; Booth, J. A. Optimisation of the hemolysis assay for the assessment of cytotoxicity. Int. J. Mol. Sci. 2023, 24, 2914 (20 pages). https://doi.org/10.3390/ijms24032914
Contact: Henrik Franzyk; henrik.franzyk@sund.ku.dk
(H. Franzyk)
Multidrug-resistant (MDR) Gram-negative bacteria cause infections only treatable with last-resort antibiotics. Overexpression of efflux pumps is a common trait of most MDR strains, and thus it is the aim to explore novel approaches for efficient bacterial delivery of efflux pump inhibitors (EPIs), capable of restoring sensitivity to antibiotics. This comprises design and synthesis of conjugates with uptake-promoting moieties, which is hypothesized to constitute a viable means to obtain such adjuvant antibiotics. A major aim is to reduce toxicity and off-target effects of the current most efficient EPIs that include drug candidates developed for treatment of other diseases. A successful project outcome is compounds that enable resensitization of MDR strains and repurposing of existing antibiotics with profound impact for patients and society. The project is funded by The Danish Research Council (as a DFF1 project grant).
PhD student: Mikkel Stampe Madsen
Contact: Henrik Franzyk; henrik.franzyk@sund.ku.dk
(P.R. Hansen)
In recent years, many bacterial pathogens have become resistant or insensitive to most of the currently available antibiotics. Especially worrying are the multidrug-resistant Gram-negative bacteria, including Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae and Acinetobacter baumannii. These are treated with the relatively toxic last-resort cyclic lipopeptide colistin, leaving no other useful therapeutic approaches. Currently, the bottleneck in cyclic lipopeptide discovery is identification of potent macrocyclic scaffolds due to their chemical complexity. Recently, we have developed a strategy to obtain small 3- to 5-membered macrocyclic peptidomimetics, as well as complex structures such as cyclic and bicyclic antimicrobial lipopeptides. They represent promising unexplored scaffolds for the synthesis of novel antimicrobial compounds of critical importance. Together with Prof. Anders Løbner Olesen (UCPH), we identified an all-D-peptide, BP214, which shows promising activity against colistin-resistant strains of A. baumannii. In continuation of this work we have synthesized , 18 C-locked analogs of BP214. Cyclization was achieved by reacting the ε-amino group of a C-terminal lysine residue with a bromoacetyl group attached to the Nαamino group of the N-terminal amino acid, generating a secondary amine at which the exocyclic lipopeptide tail was assembled. A few of the analogues showed promising activity against E. coli, A. baumannii, and P. aeruginosa. The project is funded by the Hørslev Foundation.
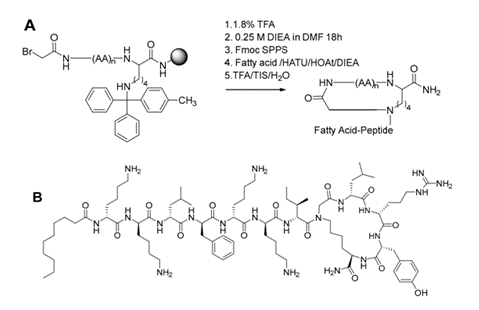 Figure: (A) General Scheme for the synthesis of C-locked peptides. (B) An example of a C-locked BP214 analog
Figure: (A) General Scheme for the synthesis of C-locked peptides. (B) An example of a C-locked BP214 analog
Selected publications:
Andersen, I. K. L.; Thomsen, T. T.; Rashid, J.; Bobak, T. R.; Oddo, A.; Franzyk, H.; Løbner-Olesen, A.; Hansen, P. R. C-locked analogs of the antimicrobial peptide BP214. Antibiotics 2022, 11, 1080 (10 pages). https://doi.org/10.3390/antibiotics11081080.
A. Oddo, L. Münzker and P. R. Hansen. Peptide macrocycles featuring a backbone secondary amine: A convenient strategy for the synthesis of lipidated cyclic and bicyclic peptides on solid support. Org. Lett.2015, 17, 2502-2505. http://dx.doi.org/10.1021/acs.orglett.5b01026
Contact: Paul Robert Hansen; prh@sund.ku.dk
(P.R. Hansen)
Cyclic AMPs are an attractive scaffold for novel antimicrobial agents. We have shown that introduction of a single flexible residue can prove useful for the antimicrobial activity and cytotoxicity. We recently described a novel class of macrocyclic peptides containing a 8-amino-3,6-dioxaoctanoic acid linker. We synthesized eighteen peptide analogues of the lead compound BSI‐9, produced in four individual stages, with a different focus in each stage; cyclization point, hydrophobicity, cationic side‐chain length, and combinations of the last two. Specifically one compound S3(B), exhibited improved activity against Staphylococcus aureus and Pseudomonas aeruginosa with MIC of 4 μg/mL and 8 μg/mL, respectively, compared to the original BSI‐9, which had an MIC of 16–32 μg/mL.
In continuation of this study, 18 cyclic lipopeptides were synthesized and characterized. It was found that introduction of fatty acids in positions next to the flexible linker was more strongly linked to antimicrobial activity. The fatty acid length altered the overall hydrophobicity, which was the driving force for both high antimicrobial and hemolytic activity. Peptides became highly hemolytic when carbon-chain length exceeded 10 (i.e., C10), overlapping with the optimum for antimicrobial activity (i.e., C8–C12). The most promising candidate (C8)5 showed antimicrobial activity corresponding to that of S3(B), but with an improved hemolytic profile.
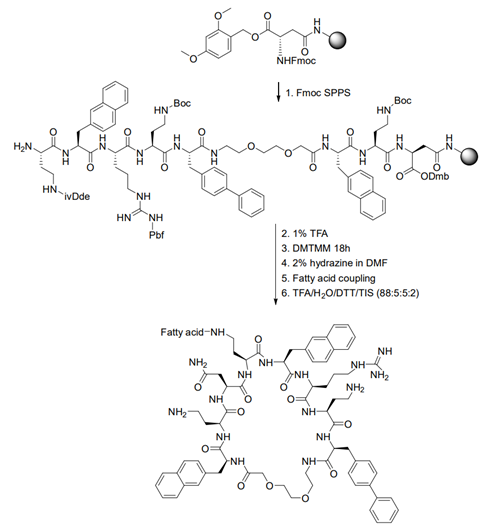
Figure: Strategy for synthesis of a fatty acid S3(B)analogue.
Selected publications:
Jensen, S.K. Jensen; Thomsen, T.T.; Oddo, A.; Franzyk, H.; Løbner-Olesen, A.; Hansen, P.R. Novel cyclic lipopeptide antibiotics: Effects of acyl chain length and position. Int. J. Mol. Sci. 2020, 21, 5829 (18 pages). https://doi.org/10.3390/ijms21165829
Thomsen, T.T.; Mendel, H.C.; Al-Mansour, W.; Oddo, A.; Løbner-Olesen, A.; Hansen, P.R. Analogues of a Cyclic Antimicrobial Peptide with a Flexible Linker Show Promising Activity against Pseudomonas aeruginosa and Staphylococcus aureus. Antibiotics 2020, 9, 366-379. https://www.mdpi.com/2079-6382/9/7/366
Oddo, A.; Thomsen, T.T.; Britt, H. M.; Løbner-Olesen, A.; Thulstrup, P.W.; Sanderson, J. M.; Hansen, P.R. Modulation of backbone flexibility for effective dissociation of antibacterial and hemolytic activity in cyclic peptides. ACS Med. Chem. Lett. 2016, 7, 741-745. https://doi.org/10.1021/acsmedchemlett.5b00400
(P.R. Hansen)
This project is in collaboration with Assoc. Prof. Peter Thulstrup (Dept. Chemistry, Univ. Copenhagen), Prof. Anders Løbner-Olesen (Dept. Biology, Univ. Copenhagen) and Prof. Niels Frimodt-Møller (Rigshospitalet, Copenhagen). Siderophores are linked to the bacterial metal iron homeostasis. The aim of this project is to conjugate antibiotics and/or antimicrobial peptides to siderophores. This could increase efficacy and alleviate toxicity issues associated with high antibiotic doses. Because individual bacteria utilize a few specific siderophores, such drugs could increase specificity to obtain antibiotics targeted for selected pathogens or strains of bacteria. Such narrow-spectrum antibiotics could act to decrease the probability of microorganisms evolving resistance - and may help to preserve the non-pathogenic microbiota.
Contact: Paul Robert Hansen; prh@sund.ku.dk
(co-PI: P.R. Hansen)
This project is a collaboration with Assoc. Prof. Reidar Lund (Univ. of Oslo, Norway) and Prof. Hårvard Jenssen (Roskilde University, Denmark). The aim of the project is to develop a methodological platform mainly based on state-of-the-art neutron scattering methods. De novo designed antimicrobial peptides and peptidomimetics are tested systematically in an iterative fashion using both the physico-chemical neutron-based techniques and biological screening assays. New insights into the formation and mode of action of antimicrobial peptide and peptidomimetic nanosheets, will be used to develop novel antimicrobial surfaces for biomedical applications against device-associated infections. The project is funded by Nordforsk.
Selected publications:
Lone, A.; Nielsen, J.E.; Thulstrup, P.W.; Lund, R.; Hansen, P.R.; Jenssen, H. Cyclic N-locked indolicidin analogues with antimicrobial activity: Effect of ring size and fatty acid acylation. Eur. J. Med. Chem. Rep. 2022, 6, 100080 (7 pages). https://doi.org/10.1016/j.ejmcr.2022.100080
Lone, A.; Thomsen, T. T.; Nielsen, J. E.; Thulstrup, P. W.; Klitgaard, R. N.; Løbner-Olesen, A.; Lund, R.; Jenssen, H.; Hansen, P. R. Structure-activity study of an all-D antimicrobial octapeptide D2d. Molecules 2019, 24, 4571 (17 pages). https://doi.org/10.3390/molecules24244571
Nielsen, J. E.; Lind, T. K.; Lone, A.; Gerelli, Y.; Hansen, P. R.; Jenssen, H.; Cárdenas, M.; Lund, R. A biophysical study of the interactions between the antimicrobial peptide indolicidin and lipid model systems. Biochim. Biophys. Acta – Biomembr. 2019, 1861, 1355-1364. https://doi.org/10.1016/j.bbamem.2019.04.003
Contact: Paul Robert Hansen; prh@sund.ku.dk
(P.R. Hansen & H. Franzyk)
In collaboration with Assoc. Prof. Peter Damborg (Dept. Veterinary and Animal Sciences, UCPH) we aim to develop antibiotics for veterinary use. Skin infections in dogs are typically caused by Staphylococcus pseudintermedius and Pseudomonas aeruginosa, which are challenging to combat due to resistance. Therefore, antimicrobial agents with restricted use in veterinary medicine are urgently needed. Interestingly, peptide-peptoid hybrid B1 showed high potency and rapid killing kinetics against clinical isolates of these bacteria.
Peptidomimetics enabling antibiotics for Gram-positive infections to be repurposed for use against Gram-negative bacteria were identified in collaboration with Prof. Luca Guardabassi (Dept. Veterinary and Animal Sciences), are these are further explored together with Assoc. Prof. Liam Good (Royal Veterinary College, London). Resistant E. coli can be sensitized to macrolides by combination with such peptidomimetics, and the reduction in the active concentrations of tilmicosin indicates a potential to manage E. coli enteritis in pigs.
Also, in collaboration with Prof. Hanne Ingmer (Dept. Veterinary and Animal Sciences) stable peptide/β-peptoid hybrids are explored as potential leads for antibacterials against Gram-positive pathogens. Intriguingly, subclasses of these peptidomimetics exhibit potent activity toward vancomycin-resistant enterococci including clinical isolates of both human and veterinary origin.
Selected publications:
Ma, Y.; Pirolo, M.; Subramani, P.; Gehring, R.; Damborg, P.; Franzyk, H.; Guardabassi, L. mSphere 2022, 7, e00402-22 (10 pages). https://doi.org/10.1128/msphere.00402-22
Vestergaard, M.; Skive, B.; Domraceva, I.; Ingmer, H.; Franzyk, H. Int. J. Mol. Sci. 2021, 22, 5617 (14 pages). https://doi.org/10.3390/ijms22115617
Greco, I.; Emborg, A. P.; Jana, B.; Molchanova, N.; Oddo, A.; Damborg, P.; Guardabassi, L.; Hansen, P. R. Sci. Rep. 2019, 9, 3679. https://doi.org/10.1038/s41598-019-39042-3
Baker, K. R Jana, B.; Hansen, A. M.; Vissing, K. J.; Nielsen, H. M.; Franzyk, H.; Guardabassi, L. Front. Cell. Infect. Microbiol. 2019, 9, 236 (13 pages). https://doi.org/10.3389/fcimb.2019.00236
Molchanova, N.; Damborg, P.; Hansen, P. R.; Franzyk, H. J. Pept. Sci. 2018, e3098 (8 pages). https://doi.org/10.1002/psc.3098
Contacts: Paul Robert Hansen; prh@sund.ku.dk and Henrik Franzyk; henrik.franzyk@sund.ku.dk
(co-PI: H. Franzyk)
In a collaboration led by Lorenzo Stella (Assoc. Prof., Univ. Rome Tor Vergata, Italy) with H. Franzyk as a key collaborator, the inoculum effect (i.e., how the active concentration of an antimicrobial agent depends on the initial cell density in growth inhibition assays) was studied for a series of antibacterial peptides (AMPs) and peptidomimetics. This effect proved most pronounced at high cell densities, and for membrane-active compounds it was rationalized by a model, hypothesizing that a threshold number of cell-bound molecules is required to induce bacterial killing, which infers that minimal inhibitory concentrations (MICs), as might be expected, gradually increase with increased bacterial density. As a continuation of this study we are now studying a number of additional AMPs.
Publication:
Loffredo, M. R.; Savini, F.; Bobone, S.; Casciaro, B.; Franzyk, H.; Mangoni, M. L.; Stella, L. Inoculum effect of antimicrobial peptides. Proc. Natl. Acad. Sci. U.S.A. 2021, 118, e2014364118 (10 pages). https://doi.org/10.1073/pnas.2014364118
Inflammatory and Autoimmune Diseases
(co-PI: P.R. Hansen)
Epitope mapping of proteins related to autoimmune diseases is carried out in collaboration with Prof. Gunnar Houen and Postdoc Nicole Trier (Rigshospitalet, Glostrup/Southern University of Denmark). The cause of autoimmune diseases is unknown, but one hypothesis is molecular mimicry, where a foreign antigen shares sequence or structural similarities with self-antigens. The aim of this project is to use autoantibodies from selected autoimmune diseases, such as Rheumatoid arthritis, to identify which protein regions they recognize. This is performed by synthesizing overlapping peptides covering the entire protein, followed by detremination of structure activity relationships (SARs) by studies of individual peptides.
Selected publications:
Kyllesbech, C.; Trier, N. H.; Mughal, F. P.; Hansen, P. R.; Holmström, M. O.; El Fassi, D.; Hasselbalch, H.; Skov, V.; Kjær, L.; Ciplys, E.; Slibinskas, R.; Frederiksen, J. L.; Højrup,P.; Houen, G. Curr. Res. Transl. Med. 2023, 71, 103380 (13 pages). https://doi.org/10.1016/j.retram.2023.103380.
Mughal, F. P.; Bergmann, A. C.; Huynh, H. U. B.; Nielsen, S.; Mansha, I.; Kesmeh, M.; Schürch, P. M.; Theocharides, A. P. A.; Hansen, P. R.; Friis, T.; Holmeren, M. Ö.; Ciplys, E.; Slibinskas, R.; Højrup, P.; Trier, N. H.; Houen, G. Int. J. Mol. Sci. 2022, 23, 6803 (21 pages). https://doi.org/10.3390/ijms23126803
Trier, N. H.; Valdarnini, N.; Fanelli, I.; Rovero, P.; Hansen, P. R.; Schafer, C.; Ciplys, E.; Slibinskas, R.; Pociot, F.; Friis, T.; Houen, G. Int. J. Mol. Sci. 2022, 23, 4424 (16 pages). https://doi.org/10.3390/ijms23084424
Fanelli, I.; Rovero, P.; Hansen, P. R.; Frederiksen, J.; Houen, G.; Trier, N. H. Antibodies 2022, 11, 20 (15 pages). https://doi.org/10.3390/antib11010020
Fanelli, I.; Rovero, P.; Hansen, P. R.; Frederiksen, J.; Houen, G.; Trier, N. H. Antibodies 2021, 10, 27 (12 pages). https://doi.org/10.3390/antib10030027
Trier, N. H.; Holm, B. E.; Hansen, P. R.; Slot, O.; Locht, H.; Houen, G. Antibodies 2019, 8, 37 (10 pages). https://doi.org/10.3390/antib8020037
Valdarnini, N.; Holm, B.; Hansen, P. R.; Rovero, P.; Houen, G.; Trier, N. Int. J. Mol. Sci. 2019, 20, 2909 (13 pages). https://doi.org/10.3390/ijms20122909
Trier, N.; Hansen, P. R.; Houen, G. Int. J. Mol. Sci. 2019, 20, 6289 (22 pages). https://doi.org/10.3390/ijms20246289
Contact: Paul Robert Hansen; prh@sund.ku.dk
(P.R. Hansen and M. Sheykhzade)
As part of an ongoing study on the mode of action for h-α-CGRP-induced vasodilation, we have developed a 9-fluorenylmethyloxycarbonyl (Fmoc)-based strategy for the solid-phase synthesis (SPPS) of h-α-CGRP and three fluorescent analogues. Thus, four peptides were synthesized by Fmoc SPPS: h-α-CGRP without tagging (i.e., wild-type CGRP) and three fluorescent analogues labelled with 5(6)-carboxyfluorescein (CF) via a 6-aminohexanoic acid (Ahx) spacer either at the N-terminus or on the side chain of Lys24or Lys35, all containing the native disulfide bond. Following SPPS and characterization, the biological activity of these peptides was tested in isolated mesenteric arteries of rat and mice, as well as in human subcutaneous arteries. The functional experiments were carried out in order to determine whether tagging of the native h-α-CGRP (i.e., wild-type CGRP) would result in loss of potency. [Lys35(Ahx-CF)]-labelled CGRP and wild-type CGRP showed similar potency, whereas [Lys24(Ahx-CF)]-labelled h-α-CGRP showed approximately 10-fold lower potency as compared to that of the wild-type α-CGRP. The N-terminally tagged α-CGRP analogue was the least potent compound in all three species.
Publication:
Zhu, J.; Pedersen, M. D.; Ahmed, L. S.; Abdolalizadeh, B.; Grell, A.-S.; Berg, J. O.; Thulstrup, P. W.; Franzyk, H.; Edvinsson, L.; Sams, A.; Sheykhzade, M.; Hansen, P. R. Fluorescent analogues of human α-calcitonin gene-related peptide with potent vasodilator activity. Int. J. Mol. Sci. 2020, 21, 1343 (15 pages). https://doi.org/10.3390/ijms21041343
Contact: Paul Robert Hansen; prh@sund.ku.dk
(H. Franzyk)
Lipidated analogs of α-peptide/β-peptoid hybrids have been designed to exert potent immunomodulatory properties, e.g., LPS-induced cytokine secretion from human leukocytes was blocked by nanomolar concentrations. Also, the pro-inflammatory response to lipoteichoic acid (a major pro-inflammatory component of Gram-positive bacteria) was neutralized.
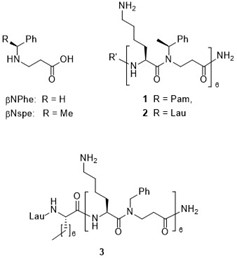 Figure. Immunomodulatory peptidomimetics
Figure. Immunomodulatory peptidomimetics
Lipidated α-peptide/β-peptoid hybrids were found to exert potent immunomodulatory properties, e.g., LPS-induced cytokine secretion from human leukocytes was blocked by nanomolar concentrations. Also, the pro-inflammatory response to lipoteichoic acid (a major pro-inflammatory component of Gram-positive bacteria) was neutralized. In collaboration with Prof. C. Dahlgren (Univ. Gothenburg), Pam-[Lys-βNspe]6-NH2 (1) was identified as an extremely potent selective inhibitor of human neutrophil activation via formyl peptide receptor 2 (FPR2). Importantly, Lau-[Lys-βNspe]6-NH2 (2) is also a potent antagonist of the mouse orthologue Fpr2. A structure-activity study identified Lau-[(S)-Aoc]-[Lys-βNPhe]6-NH2 (3) as a potent cross-species FPR2 agonist, thus establishing the first class of compounds with cross-species subtype selectivity, enabling exploration of the immunoregulatory role of FPR2 via mouse models. In collaboration with Prof. R.E.W. Hancock (Univ. Br. Columbia, Canada) such an FPR2 antagonist was found to be as potent as indomethacin in a mouse model of sterile inflammation.
Selected publications:
Bing, C.; Wu, B. C.; Skovbakke, S. L.; Hancock, R. E. W.; Franzyk, H. Front. Immunol. 2020, 11, 2102 (11 pages). https://doi.org/10.3389/fimmu.2020.02102
Holdfeldt, A.; Skovbakke, S.; Gabl, M.; Nielsen, C.; Dahlgren, C.; Franzyk, H.; Forsman, H. ACS Omega 2019, 4, 5968-5982. https://doi.org/10.1021/acsomega.9b00098
Holdfeldt, A.; Skovbakke, S. L.; Winther, M.; Gabl, M.; Nielsen, C.; Larsen, C. J.; Wang,J. M.; Karlssson, A.; Dahlgren, C.; Forsman, H.; Franzyk, H. J. Biol. Chem. 2016, 291, 19888-19899. https://doi.org/10.1074/jbc.M116.736850
Skovbakke, S. L.; Winther, M.; Gabl, M.; Holdfeldt, A.; Linden, S.; Wang, J. M.; Dahlgren, C.; Franzyk, H.; Forsman, H. Biochem. Pharmacol. 2016, 119, 56-65. https://doi.org/10.1016/j.bcp.2016.09.004
Skovbakke, S. L.; Larsen, C. J.; Heegaard, P. M. H.; Moesby, L.; Franzyk, H. J. Med. Chem. 2015, 58, 801-813. https://doi.org/10.1021/jm501341h
Contact: Henrik Franzyk; henrik.franzyk@sund.ku.dk
(co-PI: P.R.Hansen)
In a collaboration led by Associate Professor, Charlotte Rohde Knudsen, Aarhus University, cyclic peptides capable of promoting the readthrough of nonsense mutations during protein biosynthesis have been identified and further investigated. A DNA-encoded library of hexameric cyclic peptides was expressed in yeast. From this library, selection studies in vivo have identified 10 specific cyclic peptides capable of making the protein synthesis apparatus of the cells read through nonsense mutations in reporter genes. The identified cyclic peptides are potential drug candidates for treating diseases such as muscular dystrophy, cystic fibrosis, and different forms of cancer all caused by nonsense mutations. The cyclic peptide candidates have so far been expressed in cells through plasmid DNA encoding them. Chemical synthesis of the cyclic peptides will allow further mechanistic in vivo and in vitro studies
Contact: Paul Robert Hansen; prh@sund.ku.dk
Solid-Phase Synthesis
(co-PI: H. Franzyk)
Exploration of PNA-peptide conjugates as potential antisense antibiotics necessitates fast and efficient synthesis protocols to perform structure-activity relationships and in vivo studies in animal models. Most developed PNA monomers suffer from solubility issues, byproduct formation during cleavage, lack of commercial availability, or a very high price.
Therefore, we developed rapid and efficient protocols for large-scale synthesis of Fmoc/Boc-protected PNA monomers having advantageous solubility properties, full compatibility with SPPS (both manual and automated), and facilitated purification of peptide conjugates due to the traceless nature of the Boc protecting group.
Conjugation of a delivery peptide, displaying a thiol functionality (e.g., a Cys residue), with a PNA oligomer with an unprotected amine (e.g., the N-terminus or a C-terminal Lys residue) was conveniently achieved via a one-pot assembly using a bisfunctional maleimide/N-hydroxysuccinimidyl ester linker (e.g., Mal-PEG2-OSu). An optimized protocol with respect to ratios between the reactants ands recommended reaction times was deviced. Formation of a maleimide-PNA intermediate, and its conversion into a PNA-peptide conjugate could readily be followed by analytical HPLC.
Associated publications:
Hansen, A. M.; Bonke, G.; Hogendorph, W. F. J.; Björkling, F.; Nielsen, J.; Nielsen, P. E.; Kongstad, K. T.; Zabicka, D.; Franzyk. H. Eur. J. Med. Chem. 2019, 168, 134-145. https://doi.org/10.1016/j.ejmech.2019.02.024
Shaikh, A. Y.; Björkling, F.; Nielsen, P. E.; Franzyk, H. Eur. J. Org. Chem. 2021, 2792-2801. https://doi.org/10.1002/ejoc.202100278
Shaikh, A. Y.; Hansen, A. M.; Franzyk, H. 2020. Methods Mol. Biol. (Clifton, N.J.), Springer (New York, NY) (Clifton, N.J.) vol. 2105, pp. 97-118. https://doi.org/10.1007/978-1-0716-0243-0_6
Hansen, A. M.; Shaikh, A. Y.; Franzyk, H. 2020. Methods Mol. Biol. (Clifton, N.J.), Springer (New York, NY) vol. 2105, pp. 1-16. https://doi.org/10.1007/978-1-0716-0243-0_1
Contact: Henrik Franzyk; henrik.franzyk@sund.ku.dk
(H. Franzyk)
α-Peptoids as well as peptide/α-peptoid hybrids and peptide/β-peptoid hybrids constitute major classes of proteolytically stable peptidomimetics, which have been extensively investigated as mimetics of biologically active peptides. Lipidated peptide/β-peptoid hybrids, found to be promising immunomodulatory lead compounds, were selected as targets when developing protocols suitable for gram-scale solid-phase synthesis (SPS) of representatives belonging to this compounds class. This will enable extensive animal in vivo studies.
First, we examined the origin of byproducts typically appearing in crude mixtures of even relatively short N-benzylated peptide/β-peptoid oligomers. Interestingly, we found that they arise from their inherent low stability under prolonged exposure to strongly acidic conditions used for simultaneous deprotection and cleavage of the peptidomimetics from the linker on a resin. These byproducts were identified as partially N-debenzylated oligomers.
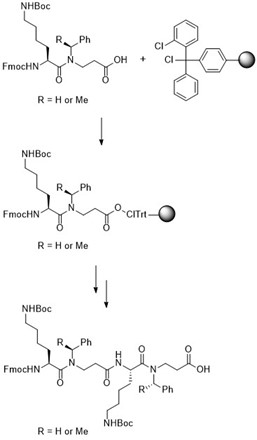
Figure. Solid-phase synthesis of tetrameric building blocks
Our initial work on assembly of peptide/β-peptoid oligomers with an alternating design had established that it was beneficial to form the amide bond between the carboxyl group of an α-amino acid and the congested amino functionality of the β-peptoid residue via a solution-phase coupling. To further simplify oligomer assembly on solid phase, we developed a protocol for purification-free SPS of tetrameric building blocks. These were applied in manual SPS of 12-mer peptidomimetics, and they were found to provide more readily purified crude products as compared to those obtained by using dimeric building blocks. Moreover, the tetrameric building blocks could be utilized in automated SPS with microwave-assisted heating.
Associated publication:
Hansen, A. M.; Skovbakke, S. L.; Christensen, S. B.; Gassol, I. P.; Franzyk, H. Studies on improved large-scale synthesis of peptide-peptoid hybrids: ligands for formyl peptide receptors. Amino Acids 2019, 51, 205-218. https://doi.org/10.1007/s00726-018-2656-x
Contact: Henrik Franzyk; henrik.franzyk@sund.ku.dk
(H. Franzyk)
Several potential therapeutics display guanidino groups, and therefore efficient guanidinylation reagents are vital to their easy access. As part of our ongoing studies of guanidinylated delivery moieties, we performed a comparative study examining possible advantages of pyrazole- and triazole-based reagents (i.e., 1 and 2 versus 3 and 4).
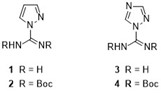 Figure. Guanidinylation reagents.
Figure. Guanidinylation reagents.
A gram-scale synthesis of 1H-triazole-1-[N,N′-bis(tert-butoxycarbonyl)]carboxamidine (4) enabled a thorough study of its reactivity. Assessment of reactivity of guanidinylation reagents was conveniently performed in deuterated solvents (DMF-d7 or THF-d8), enabling continuous monitoring by 1H and 13C NMR spectroscopy. Reagent 4 exhibited superior performance in solution-phase conversions, and it also proved versatile in on-resin transformations with short reaction times when employing two equivalents per amino group. Complete guanidinylation of sterically hindered N-terminal residues in resin-bound peptides was readily achieved. Extended utility of 4 was demonstrated in automated peptide synthesis with MW-assisted or induction-based heating.
Efficient and selective removal of a temporary protecting group is often difficult. Commercially available Nα-Fmoc-Nε-(4-methyltriphenylmethyl) (Mtt)-protected lysine is extensively used for on-resin modification of resin-bound peptide intermediates. A reliable protocol for on-resin removal of Mtt groups in the presence of tert-butyloxycarbonyl (Boc) groups was needed. Typically, 1-3% trifluoroacetic acid (TFA) in dichloromethane (DCM) are employed, but often premature removal of Boc groups occurs. A head-to-head comparison of a wide range of acids in DCM revealed that convenient Mtt deprotection conditions are successive treatments with 30% hexafluoroisopropanol or 30% perfluoro-tert-butanol.
Associated publications:
Wester, A.; Björkling, F.; Franzyk, H. J. Org. Chem. 2021, 86, 14371-14380. https://doi.org/10.1021/acs.joc.1c00994
Wester, A.; Hansen, A. M.; Hansen, P. R.; Franzyk, H. Amino Acids 2021, 53, 1455-1466. https://doi.org/10.1007/s00726-021-03059-8
Contact: Henrik Franzyk; henrik.franzyk@sund.ku.dk
(co-PI: H. Franzyk)
Since cells in solid tumors divide less rapidly than cells in the bone marrow and immune cells, inhibitors of mitose cause severe side effects when used as therapy. One approach to overcome this is to develop drugs based on cytotoxins (e.g., thapsigargin) that kill cells in all phases of the cell cycle, but this requires targeting to the malignant tissue.
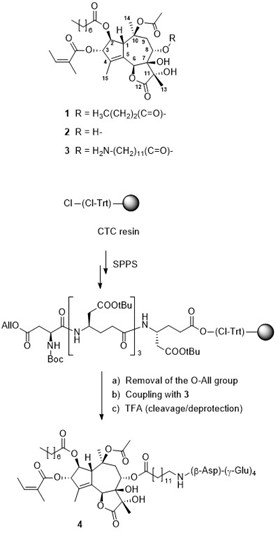 Figure. Solid-phase synthesis of mipsigargin (4).
Figure. Solid-phase synthesis of mipsigargin (4).
For thapsigargin (1) selectivity for tumor-associated cells may be achieved (upon conversions 1 ® 2 ® 3) by conjugation to a peptide undergoing selective cleavage in vicinity of tumors. A solid-phase synthesis protocol was developed for three validated prodrugs cleavable by human kallikrein 2, prostate-specific antigen, or prostate-specific membrane antigen (i.e., mipsigargin), respectively. Mipsagargin (4) was synthesized from the corresponding resin-bound peptide (i.e., tBu-protected H-β-Asp-[γ-Glu]3-Glu-OH) and a 12-aminododecanoyl derivative of thapsigargin.
Likewise, targeting cytotoxic 4β-phorbol esters toward cancer tissue was attempted by conjugating a 4β-phorbol derivative with a peptide substrate for a protease expressed by cancer cells. Cleavage of the peptide part in the vicinity or within tumors was envisioned to selectively release the lipophilic cytotoxin. Cellular assays revealed the expected activation of PKC by the prodrugs, however, efficient killing of both peptidase-positive and peptidase-negative cells was found. Consequently, the intact prodrug was also internalized into benign cells.
These studies were performed as a collaboration with Prof. Søren B. Christensen and visiting PhD student Tomáš Zimmermann (Univ. Chemistry and Technology, Prague, Czech Republic).
Associated publications:
Tarvainen, I.; Zimmermann, T.; Heinonen, P.; Jäntti, M. Yli-Kauhaluoma, J.; Talman, V.; Franzyk, H.; Tuominen, R. Christensen, S. B. ACS Med. Chem. Lett. 2019, 11, 671-677. https://doi.org/10.1021/acsmedchemlett.9b00554
Zimmermann, T.; Christensen, S. B.; Franzyk, H. Molecules 2018, 23, e1463. https://doi.org/10.3390/molecules23061463
Contact: Henrik Franzyk; henrik.franzyk@sund.ku.dk
Drug Delivery and Vaccines
(Prof. C. Foged, Dept. Pharmacy, Univ. Copenhagen; co-PI H. Franzyk)
A delivery technology based on well-tolerated lipidoid-polymer hybrid nanoparticles (LPNs) with an unprecedented ability to deliver nucleic acids to target tissues has been invented. Lipidoids comprise a novel class of cationic lipid-like compounds, capable of complexing polyanionic nucleic acids via attractive electrostatic interactions, with subsequent mediation of cellular uptake, endosomal escape and cytosolic delivery. PLGA serves as a polymeric core component conferring sustained release properties to the nanoparticles. LPNs can silence key genes involved in inflammation, and we hypothesize that the use of lipidoids as mRNA transfection agents may have an added benefit for vaccination purposes due to their TLR4 agonist activity.
Selected publications:
Aljabbaria, A.; Lokras, A. G.; Kirkensgaard, J. J. K.; Rades, T.; Franzyk, H.; Thakur, A.; Zhang, Y.; Foged, C. J. Colloid Interface Sci. 2023, 633, 907-922. https://doi.org/10.1016/j.jcis.2022.11.141
Xu, Y.; Parra-Ortiz,E.; Wan, F.; Cañadas, O.; Garcia-Alvarez, B.; Thakur, A.; Franzyk, H.; Pérez-Gil, J.; Malmsten, M.; Foged, C. J. Colloid Interface Sci. 2023, 633, 511-525. https://doi.org/10.1016/j.jcis.2022.11.059
Xu, Y.; Harinck, L.; Lokras, A. G.; Gerde, P.; Selg, E.; Sjöberg, C.-O.; Franzyk, H.; Thakur, A.; Foged, C. Int. J. Pharm. 2022, 621, 121758 (14 Pages). https://doi.org/10.1016/j.ijpharm.2022.121758
Xu, Y.; Turan, E. T.; Shi, Z.; Franzyk, H.; Thakur, A.; Foged, C. Front. Drug Deliv. 2022, 2, 945459 (15 pages). https://doi.org/10.3389/fddev.2022.945459
Contact: Henrik Franzyk; henrik.franzyk@sund.ku.dk
Group members
| Name | Title | Phone | |
|---|---|---|---|
| Adamsen (Wan), Uraiwan Ngamrabiab | Laboratory Technician | +4535336685 | |
| Franzyk, Henrik | Associate Professor | +4535336255 | |
| Hansen, Paul Robert | Associate Professor | +4535336625 | |
| Simonsen, Birgitte | Laboratory Technician. | +4535336148 |