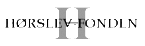Strømgaard Lab
In the Strømgaard lab, we combine tools from chemistry and biology, and apply these in peptide and protein engineering. Our aims are to develop modulators for protein-protein interactions and to provide molecular-level insight herein. We are interested in protein-protein interactions that are therapeutically relevant, in particular between integral membrane proteins and their intracellular protein partners, also known as receptor-complexes.
Targeting Protein-Protein interactions
We combine solid-phase peptide synthesis, organic chemical synthesis, and peptide microarrays with protein expression, biochemical assays, and biophysical techniques in order to develop modulators of protein-protein interactions. Examples include development of dimeric peptide-based inhibitors of PSD-95, and super-binding peptides targeting gephyrin.
Molecular Details of Protein-Protein Interactions
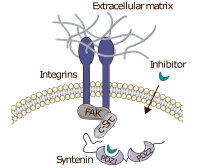
We employ peptide microarrays, incorporation of unnatural amino acids and protein semi-synthesis to address molecular determinants of protein-protein interactions, for instance regulation of interactions by post-translational protein modification. Examples include site-specific introduction of cross-linking amino acids, backbone modifications, and post-translational modifications, in particular site-specific phosphorylation, of therapeutically relevant proteins.
Our group employs peptide and protein engineering to study the molecular details of protein-protein interactions (PPIs), and develop and design specific inhibitors for these interactions. We develop in vivo active peptide-based inhibitors of PPIs with putative therapeutic relevance. In parallel, we also study the molecular mechanisms of these PPIs, including the engagement of hydrogen-bonding networks, and regulation of PPIs by post-translational modifications (PTMs). Our current projects comprise:
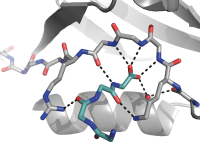
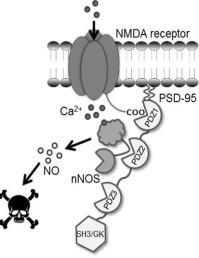
Postsynaptic density protein 95 (PSD-95) is a key protein in the postsynaptic density binding several membrane proteins, such as the glutamate NMDA receptor, and stargazin – an auxiliary subunit of AMPA receptors. PSD-95 has been a focus in the lab for several years, leading to the successful development of a high-affinity dimeric peptide inhibitor. Our spinout company, Avilex Pharma, currently pursues the lead candidate, AVLX-144, as a novel treatment for ischemic stroke. Ongoing efforts comprise development and application of radiolabeled dimeric ligands for PET imaging, generation of peptide microarrays to elucidate the PSD-95 interactome, development of novel ligands using mirror image phage display, and investigation into the effect of site-specific phosphorylation by applying unnatural amino acid mutagenesis (protein site) and peptide microarrays (ligand site)
Gephyrin is an important player in the inhibitory synapse that binds and regulates GABAA and glycine receptors. Similar to PSD-95, we developed dimeric inhibitors of gephyrin. In addition, we also developed labeled super-binding peptides that we use to visualize and modulate the inhibitory post-synapse. Ongoing efforts include application of peptide microarrays to decipher receptor-gephyrin interactions, and to elucidate the gephyrin interactome.
G proteins are central mediators of intracellular signaling of G protein-coupled receptors (GPCRs). There is growing interest in compounds that modulate the activity of G proteins. We have been using our expertise in chemical and synthetic biology to generate pharmacological tool compounds that serve as templates for the development of therapeutic substances. Our efforts led to the total synthesis of cyclic depsipeptide natural products, i.e. YM-254890 and FR900359, and subsequent structure-activity relationship (SAR) studies of these selective G protein inhibitors.
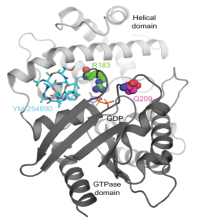
We are continuously exploring novel targets, both in-house and through collaborative efforts. Currently, we are targeting syntenin-1, which binds to a range of intracellular proteins and is an emerging cancer target. Furthermore, we investigate the munc-18 interaction (MINT) proteins that are crucial regulators of amyloid-β formation as a potential target for Alzheimer’s disease. Moreover, we study the potassium channel tetramerization domain (KCTD) protein that binds and modulates several key functions of the GABAB receptor.
2021
C. R. O. Bartling, T. M. T. Jensen, S. M. Henry, A. L. Colliander, V. Sereikaitė, M. Wenzler, P. Jain, H. M. Maric, K. Harpsøe, S. W. Pedersen, L. S. Clemmensen, L. M. Haugaard-Kedström, D. E. Gloriam, A. Ho, K. Strømgaard, Targeting the APP-Mint2 protein-protein interaction with a peptide based-inhibitor reduces amyloid-β formation, J. Am. Chem. Soc. 2021, 143, 891−901, DOI: 10.1021/jacs.0c10696
L. Haugaard-Kedström, L. S. Clemmensen, V. Sereikaite, Z. Jin, E. F. A. Fernandes, B. Wind, F. Abalde-Gil, J. Daberger, M. Vistrup-Parry, D. Aguilar-Morante, R. Leblanc, A. L. Egea Jimenez, M. Albrigtsen, K. Jensen, T. M. T. Jensen, Y. Ivarsson, R. Vincentelli, P. Hamerlik, J. Andersen, P. Zimmermann, W. Lee, K. Strømgaard, A high-affinity peptide ligand targeting syntenin inhibits glioblastoma, J. Med. Chem. 2021, 64, 1423-1434, DOI: https://doi.org/10.1021/acs.jmedchem.0c00382
M. Vistrup-Parry, W. B. Sneddon, S. Bach, K. Strømgaard, P. A. Friedman,T. Mamonova, Multisite NHERF1 phosphorylation controls GRK6A regulation of hormone-sensitive phosphate transport, J. Biol. Chem. 2021, 296, 100473, DOI: 10.1016/j.jbc.2021.100473
D. J. Essig, J. R. Balboa, K. Strømgaard, Development of peptide-based PDZ domain inhibitors, Methods Mol. Biol. 2021, 2256, 157-177.
C. Kossmann, S. Ma, L. S. Clemmensen, K. Strømgaard, Chemical synthesis of PDZ domains, Methods Mol. Biol. 2021, 2256, 193-216.
T. M. T. Jensen, C. R. O. Bartling, O. A. Karlsson, E. Åberg, L. Haugaard-Kedström, K. Strømgaard, P. Jemth, Molecular details of a coupled binding and folding reaction between the amyloid precursor protein and a folded domain, ACS Chem. Biol. 2021, 16, 1191-1200.
Z. Najarzadeh, J. Nielsen, A. Farzadfard, V. Sereikaite, K. Strømgaard, R. Meyer, D. E. Otzen, Daniel, Human Fibrinogen inhibits amyloid assembly of most Phenol Soluble Modulins (PSM) from Staphylococcus aureus, ACS Omega 2021, 6, 21960-21970.
Z. Najarzadeh, M. Zaman, V. Sereikaite, K. Strømgaard, M. Andreasen, D. E. Otzen, Daniel, Heparin promotes fibrillation of most phenol-soluble modulin virulence peptides from Staphylococcus aureus, J. Biol. Chem. 2021, 297, 100953.
M. Vistrup-Parry, X. Chen, T. L. Johansen, S. Bach, S. C. Buch-Larsen, C. R. O. Bartling, C. Ma, L. S. Clemmensen, M. L. Nielsen, M. Zhang, K. Strømgaard, Site-specific phosphorylation of PSD-95 dynamically regulates the post-synaptic density as observed by phase separation, iScience, 2021, 24, 103268.
2020
H. Zhang, A. L. Nielsen, K. Strømgaard, Recent achievements in developing selective Gq inhibitors. Med. Res. Rev. 2020, 40, 135-157.
S. Ma, K. Strømgaard, L. S. Clemmensen, Site-specific phosphorylation of PDZ domains, Methods Mol. Biol. 2020, 2133, 235-261.
E. F. A. Fernandes, L. M. Haugaard-Kedström, K. Strømgaard, Effects of a combination of lipidation and cell-penetrating peptide on a peptide dimeric inhibitor of PSD-95, Austr. J. Chem. 2020, 73, 307-311.
C. B. Borg, N. Braun, S. A. Heusser, Y. Bay, D. Weis, I. Galleano, C. Lund, W. Tian, L. M. Haugaard-Kedström, E. P. Bennett, T. Lynagh, K. Strømgaard, J. Andersen, S. A. Pless, Mechanism and site of action of big dynorphin on ASIC1a, Proc. Natl. Acad. Sci. USA 2020, 117, 7447-7454.
N. R. Christensen, M. De Luca, M. B. Lever, M. Richner, A. B. Hansen, K. L. Jensen, M. Rathje, G. Noes-Holt, D. B. Jensen, S. Erlendsson, C. R. O. Bartling, I. Ammendrup-Johnsen, S. E. Pedersen, M. Schönauer, K. B. Nissen, S. R. Midtgaard, K. Teilum, L. Arleth, A. T. Sørensen, A. Bach, K. Strømgaard, C. F. Meehan, C. B. Vægter, U. Gether, K. L. Madsen, A high-affinity, bivalent PDZ domain inhibitor complexes PICK1 to alleviate neuropathic pain, EMBO Mol. Med. 2020, 12, e11248.
J. Li, Y. Ge, J. X. Huang, K. Strømgaard, X. Zhang, X. F. Xiong, Heterotrimeric G proteins as therapeutic targets in drug discovery, J. Med. Chem. 2020, 63, 5013-5030.
G. Langlhofer, N. Schaefer, H. Maric, A. Keramidas, Y. Zhang, P. Baumann, R. Blum, U. Breitinger, K. Strømgaard, A. Schlosser, M. Kessels, D. Koch, B. Qualmann, H.-G. Breitinger, J. Lynch, C. Villmann, A novel glycine receptor variant with startle disease affects syndapin I and glycinergic inhibition, J. Neurosci. 2020, 40, 4954-4969.
A. Toto, S. Ma, F. Malagrinò, L. Visconti, L. Pagano, K. Strømgaard, S. Gianni, Comparing the binding properties of peptides mimicking the envelope protein of SARS-CoV and SARS-CoV-2 to the PDZ domain of the tight junction-associated PALS1 protein. Protein Sci. 2020, 29, 2038-2042.
M. Kristensen, K. Kucharz, E. F. A. Fernandes, K. Strømgaard, M. S. Nielsen, H. C. C. Helms, A. Bach, M. U. Tofte-Hansen, B. I. A. Garcia, M. Lauritzen, B. Brodin, Conjugation of therapeutic PSD-95 inhibitors to the cell-penetrating peptide Tat affects blood-brain barrier adherence, uptake, and permeation. Pharmaceutics 2020, 12, E661.
M. I. Rosenbaum, L.S. Clemmensen, D. S. Bredt, B. Bettler, K. Strømgaard, Targeting receptor complexes: A new dimension in drug discovery, Nature Rev. Drug Discov. 2020, 19, 884–901.
2019
X-F. Xiong, H. Zhang, M. W. Boesgaard, C. R. Underwood, H. Bräuner-Osborne, K. Strømgaard, Structure-activity relationship studies of the natural product Gq/11 protein inhibitor YM-254890, Chemmedchem, 2019, 14, 865-870.
D. Malfacini, J. Patt, S. Annala, K. Harpsøe, F. Eryilmaz, R. Reher, M. Crüsemann, W. Hanke, H. Zhang, D. Tietze, D. E. Gloriam, H. Bräuner-Osborne, K. Strømgaard, G. M. König, A. Inoue, J. Gomeza, E. Kostenis, Rational design of a heterotrimeric G protein α subunit with artificial inhibitor sensitivity. J. Biol. Chem. 2019, 294, 5747-5758.
A. Bach, B. H. Clausen, L. K. Kristensen, M. G. Andersen, D. G. Ellman, P. B. L. Hansen, H. Hasseldam, M. Heitz, D. Özcelik, E. J. Tuck, M. V. Kopanitsa, S. G. N. Grant, K. Lykke-Hartmann, F. F. Johansen, K. L. Lambertsen, K. Strømgaard, Selectivity, efficacy and toxicity studies of UCCB01-144, a dimeric neuroprotective PSD-95 inhibitor. Neuropharmacology, 2019, 150, 100-111.
N. R. Christensen, J. Čalyševa, E. F. A. Fernandes, S. Lüchow, L: S. Clemmensen, L. M. Haugaard-Kedström, K. Strømgaard, PDZ domains as drug targets. Adv. Ther. 2019, 2, 1800143.
J. Petersen, K. Strømgaard, B. Frølund, C. Clemmensen, Designing poly-agonists for treatment of metabolic diseases: Challenges and opportunities, Drugs, 2019, 79, 1187-1197.
V. Sereikaite, T. Fritzius, V. B. Kasaragod, N. Bader, H. M. Maric, H. Schindelin, B. Bettler, K. Strømgaard, Targeting the γ-aminobutyric acid type B (GABAB) receptor complex: Development of inhibitors targeting the K+ channel tetramerization domain (KCTD) containing proteins/GABAB receptor protein-protein interaction, J. Med. Chem. 2019, 62, 8819-8830.
Z. Najarzadeh, H. Mohammad-Beigi, J. N. Pedersen, G. Christiansen, T. V. Sønderby, S. A. Shojaosadati, D. Morshedi, K. Strømgaard, G. Meisl, D. Sutherland, J. S. Pedersen, D. E. Otzen, Plant polyphenols inhibit functional amyloid and biofilm formation in Pseudomonas strains by directing monomers to off-pathway oligomers, Biomolecules, 2019, 9, E659.
2018
N. G. Nørager, M. H. Poulsen, K. Strømgaard, Controlling Ca2+ permeable α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors with photochromic ion channel blockers, J. Med. Chem. 2018, 61, 8048-8053.
V. Sereikaitė, T. M. T. Jensen, C. R. O. Bartling, P. Jemth, S. A. Pless, K. Strømgaard, Probing backbone hydrogen bonds in proteins by amide-to-ester mutations. Chembiochem, 2018, 19, 2136-2145.
T. M. T. Jensen, L. Albertsen, C. R. O. Bartling, L. M. Haugaard-Kedström, K. Strømgaard, Probing the Mint2 protein-protein interaction network relevant to the pathophysiology of Alzheimer's disease. Chembiochem, 2018, 19, 1119-1122.
A. Bleem, G. Christiansen, D. J. Madsen, H. Maric, K. Strømgaard, J. D. Bryers, V. Daggett, R. L. Meyer, D. E. Otzen, Protein engineering reveals mechanisms of functional amyloid formation in Pseudomonas aeruginosa biofilms. J. Mol. Biol. 2018, 430, 3751-3763.
H. Zhang H, A. L Nielsen, M. W. Boesgaard, K. Harpsøe, N. L. Daly, X. F. Xiong, C. R. Underwood, L. M. Haugaard-Kedström, H. Bräuner-Osborne, D. E. Gloriam, K. Strømgaard, Structure-activity relationship and conformational studies of the natural product cyclic depsipeptides YM-254890 and FR900359. Eur. J. Med. Chem. 2018, 156, 847-860.
Key technologies the Strømgaard lab comprise solid-phase peptide synthesis (SPPS), protein expression and purification, and chemical protein ligation. In addition, we employ a wide range of state-of-the-art technologies for modern peptide and protein engineering:
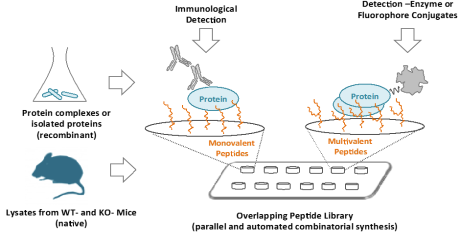
Peptide microarrays, which display hundreds of designed peptides, are used to identify binding epitopes, to perform deep mutational scanning, and to exam effects of post-translational modification (PTM). We have used peptide microarrays to develop super-binding peptides for a number of therapeutically relevant proteins, and to identify new phosphorylation sites that are important for protein-protein interactions (PPIs) in cell-surface receptors.
Picture below:
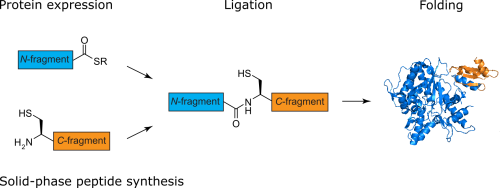
Protein semisynthesis or expressed protein ligation combine SPPS and protein expression to generate proteins endowed with any imaginable modification. We have used this technique to introduce amide-to-ester backbone modifications into proteins in order to investigate backbone hydrogen bonds and site-specific phosphorylation, and the impact of these PTMs on PPIs.
Picture below:
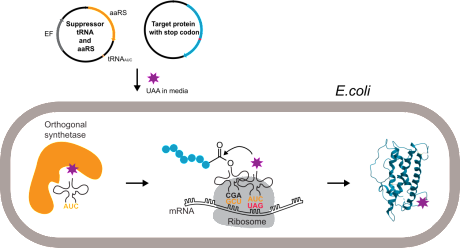
Unnatural amino acid mutagenesis or nonsense suppression mutagenesis allows site-specific introduction of non-coded amino acids into proteins by exploiting the natural protein translation machinery. We used this technology to introduce crosslinking amino acids into proteins in order to determine ligand binding sites. Currently, our efforts focus primarily on site-specific phosphorylations of intracellular adapter and scaffolding proteins.
Picture below:
We combine these technologies with a variety of biophysical methods, in particular fluorescence polarization (FP) and isothermal titration calorimetry (ITC). Further, we investigate ligands and binding epitopes in pull down studies (combined with mass spectrometry) and examine lead compounds in our in vitro absorption, distribution, metabolism, and excretion (ADME) platform.
Master and scholarship students
If you are interested in undertaking a Master ("Speciale") or scholarship project in our group, please contact Kristian Strømgaard for an informal talk about opportunities. Master students are typically doing a 6 or 12 month laboratory-based project for their final MSc thesis. A scholarship project is typically undertaken prior to a Master thesis and can last for 6–12 months, which can be combined with a Master project. We also welcome international exchange students, such as ERASMUS students. Students contribute directly to our core research projects and the majority of results from scholar and master student projects are published in peer-reviewed journals.
PhD students
When available, fully funded PhD scholarships will be posted on https://employment.ku.dk/phd/. Please apply using the online system, as e-mailed applications will not be considered. If you consider applying for a Faculty PhD stipend at the Faculty of Health and Medical Sciences or join as a visiting PhD student contact Kristian Strømgaard.
Postdoctoral fellows
Motivated and highly qualified candidates for postdoctoral positions are welcome to contact us. Postdoctoral candidates with fellowship support should send their CV and letters of recommendation to Kristian Strømgaard. When available, fully funded postdoctoral fellowships will be posted on https://employment.ku.dk/. Please apply using the online system, as e-mailed applications will not be considered.
Associate professors
Linda Haugaard-Kedström (2015-18)
Assistant professors
Dennis Özcelik (2017-20)
Hans M. Maric (2014-17)
Xiaofeng Xiong (2013-16)
Anders Bach (2013-15)
Jacob Andersen (2014-16)
Post docs
Søren W. Pedersen (2013-16)
Lena Sørensen (2011-15)
Louise Albertsen (2013-15)
Jacob Andersen (2009-13)
Mette H. Poulsen (2013-14)
Jonas N. N. Eildal (2013-14)
Nicolai Stuhr-Hansen (2007-13)
Anders Bach (2009-12)
Simon Lucas (2008-09)
Kolja M. Knapp (2004, 2008-09)
Jared K. Nelson (2005-07)
Christopher J. Armishaw (2006-07)
Stine B. Vogensen (2003)
PhD students
Vita Sereikaite (2016-19)
Christian Bartling (2015-18)
Eduardo A. F. Fernandes (2015-18)
Thomas Marker Jensen (2015-18)
Hang Zhang (2014-17)
Hafsteinn Rannversson (2014-17)
Theis S. Wilbek (2011-15)
Pella Söderhielm (2010-14)
Christel B. Jensen (2010-14)
Klaus B. Nissen (2010-14)
Niels G. Nørager (2010-13)
Louise Albertsen (2009-13)
Jonas N. N. Eildal (2009-13)
Mette H. Poulsen (2009-13)
Søren W. Pedersen (2009-13)
Lena Sørensen (2008-11)
Marie Gottschalk (2007-11)
Mette S. Jensen (2006-11)
Jacob Andersen (2006-09)
Anders Bach (2005-09)
Julie G. Rannes (2004-08)
Research assistants
Sofie A. Bach (2019)
Thea List Johansen (2019)
Daniela Danková (2018)
Griffin Moran (2015-16)
Julie Jung Andersen (2013)
Marc Heitz (2012-13)
Anders Ruskov-Nielsen (2012)
Stine Bjergegaard Pedersen (2011)
Louise Anker (2011)
Rasmus Deeskamp (2010)
Tinna B. Bach (2009-10)
Christine A. Ussing (2009)
Søren W. Pedersen (2008-09)
Sidsel U. Frølund (2007-08)
Lars S. Jensen (2003-04)
MSc students
Alexander D. Davis (2018-19)
Nichlas Mathias Welcher Karer (2018-19)
Flora Alexopoulou (2018-19)
Anna Colliander (2016-18)
Alexander Lund Nielsen (2016-17)
Rain Hindrimäe (2015-16)
Bianca Wind (2015-16)
Vita Sereikaite (2015-16)
Thomas Marker Jensen (2014-15)
Alejandro Aguayo Orozco (2014-15)
Thomas Breum Pedersen (2014-15)
Silvia Inderbinen (2014-15)
Lina Bartels (2013-14)
Christian Bartling (2013-14)
Flor Abalde Gil (2013-14)
Danyang Wang (2013-14)
Line Ryaa Nielsen (2014)
Begard Fallahi (2013-14)
Charlotte Stenum-Berg (2013-14)
Rama Hussein (2013)
Nanna Søndergaard Jeppesen (2012-13)
Anne Nielsen (2012-13)
Line Skov Nielsen (2012-13)
Hafsteinn Rannversson (2012-13)
Hannah Wapenaar (2012-13)
Julie Jung Andersen (2012-13, Novo Nordisk A/S)
Kristoffer Ringsted (2011-12)
Rune W. Christensen (2011-12)
Mark Bonefeld Nielsen (2011-12, U. Texas, Austin)
Sinan Kilic (2011-12, Princeton U.)
Anne Callesen (2011-12, IMB, Brisbane)
Anders Ruskov-Nielsen (2011-12, IMB, Brisbane)
Helle Bakke Krog (2011-12)
Kaspar Jensen (2011)
Pamela Wilson (2011)
Stine Bjergegaard Pedersen (2010-11)
Stinna M. R. Hansen (2010-11)
Søren L. Steffensen (2010-11, IMB, Brisbane)
Louise Anker (2010-11, IMB, Brisbane)
Theis Wilbek (2010-11, ETH, Zürich)
Troels Eskildsen (2010)
Mette Thomsen (2010)
Rasmus Deeskamp (2010)
Signe Egstrand Andersen (2009-10)
Mie Vindahl Andersen (2009-10)
Tinna Bach (2009-10)
Anne Barslund (2009-10)
Klaus B. Nissen (2009-10, IMB, Brisbane)
Andreas L. Ertbjerg (2009-10, TPIMS, Florida)
Amir A. Hashtroudi (2009)
Mette H. Poulsen (2009)
Linda G. Zachariassen (2009)
Louise Albertsen (2007-09, Rockefeller U.)
Julie K. Klint (2008-09, IMB, Brisbane)
Christine Ussing (2008-09, IMB, Brisbane)
Jonas N. N. Eildal (2008-09)
Jacob A. D. Clausen (2008-09)
Miriam Grønlund Pedersen (2008-09)
Gar Fai Pang (2008)
Martin Røder (2008)
Søren Toubro (2008)
Lena Sørensen (2007-08, IMB, Brisbane)
Lena Larsen (2007-08)
Anja Nielsen (2007)
Thomas B. Olsen (2007)
Søren W. Pedersen (2007)
Morten V. Andersen (2007)
Hans Chr. Troelsen (2007)
Klaus Bitsch-Jensen (2006-07, Columbia U.)
Klaus Kofoed (2006-07, Columbia U.)
Ronnie Jensen (2006)
Tina Nielsen (2006)
Nasreen Begum (2004-05)
Trine F. Andersen (2004)
BSc/Scholar students
Michèle Schönauer (2011-12)
Sinan Kilic (2011)
Mark B. Nielsen (2010, H. Lundbeck)
Claudius Wenzler (2009)
Theis Wilbek (2009)
Jonas N. N. Eildal (2007, H. Lundbeck)