BUNCH Group
We are committed to research in both medicinal chemistry and organic chemistry. While our medicinal chemistry projects focus on design and synthesis of neuroactive ligands as novel tool compounds and/or potential drug candidates, our organic chemistry projects aim to discover new synthetic methodology relevant to medicinal chemistry.
We take a creative approach and combine state-of-the-art computational techniques to design new valuable tool compounds for in vitro and in vivo studies in the CNS. We work closely with pharmacologists at the department and around the world to achieve these goals! Our work span several targets:
- Ionotropic glutamate receptors (iGluRs)
- First GluK5-selective ligand: submitted for publication 2024
- First GluK3-selective antagonist: ACS Chem Neurosci 2022
- Proline analogs: ACS ChemNeurosci 2020 - and references therein
- Metabotropic glutamate receptors (mGluRs)
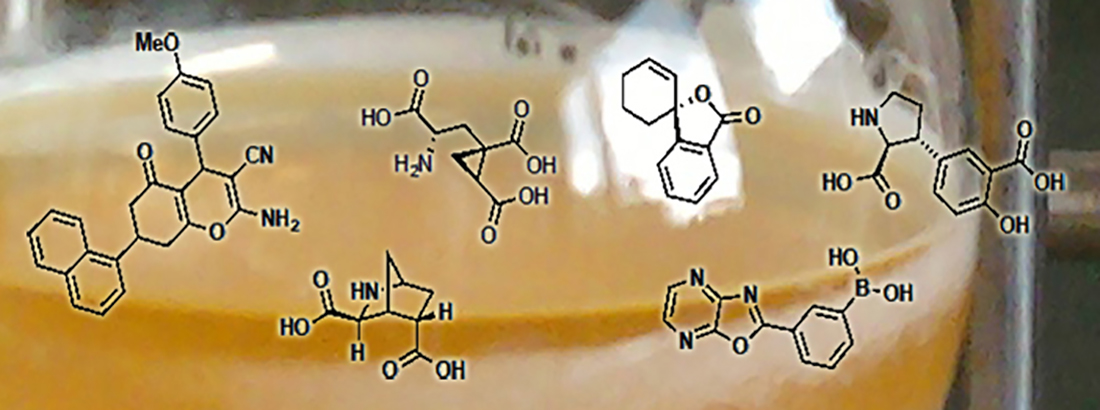
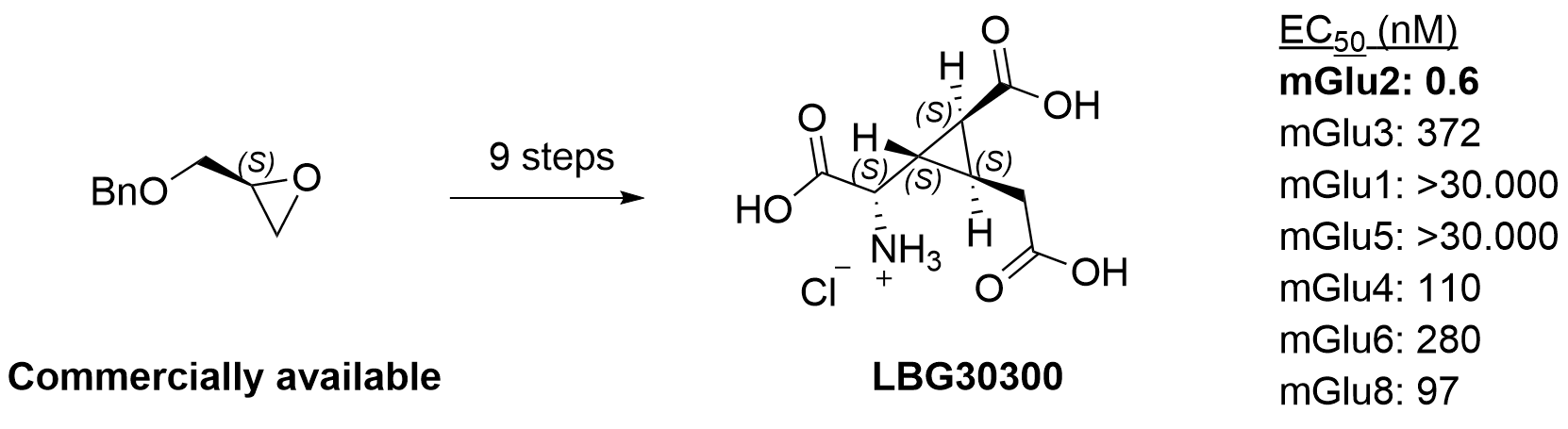
- Picomolar and selective mGlu2 agonist LBG30300: J Med Chem 2024
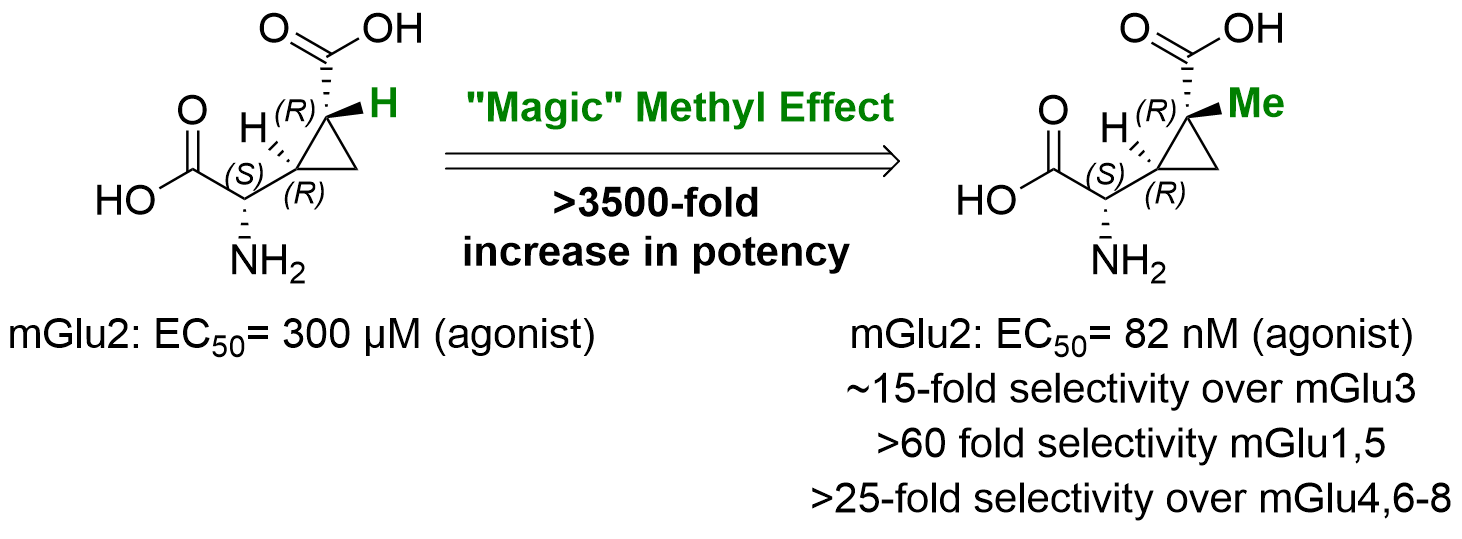
- Introducing a "MAGIC" methyl group, LBG30120: Eur J Med Chem 2024
- Picomolar and selective mGlu2 agonist LBG30300: J Med Chem 2024
- Excitatory amino acid transporters (EAATs)
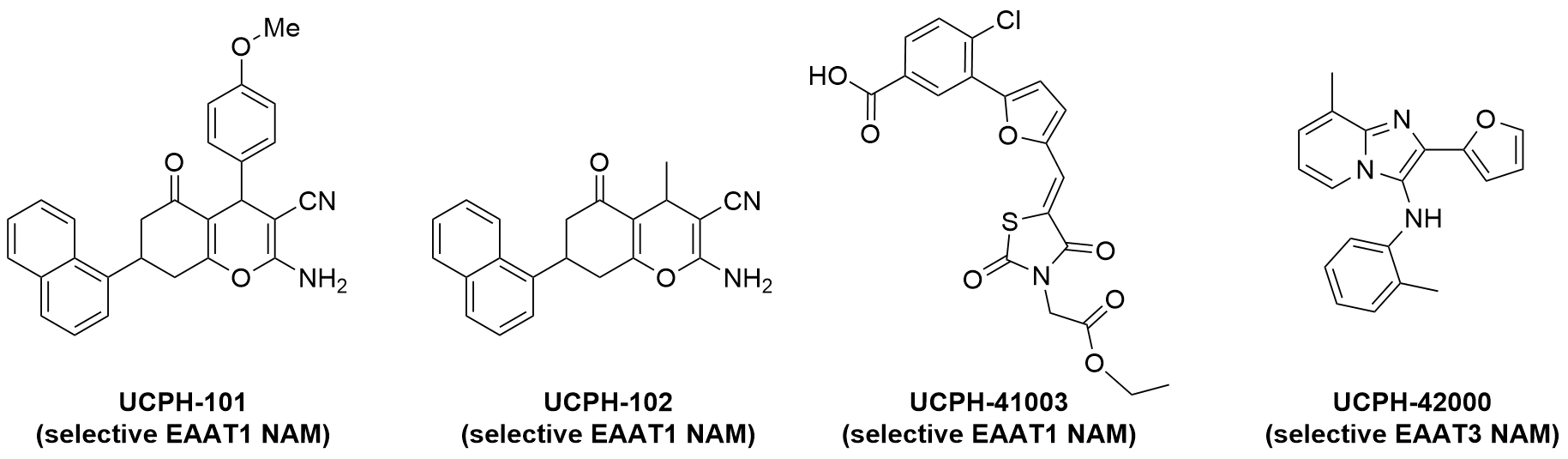
- Selective EAAT1 NAM, UCPH-101: J Med Chem 2010
- Selective EAAT1 NAM, UCPH-102: ChemMedChem 2016
- Selective EAAT1 NAM, UCPH-41003: J Med Chem 2016
- Selective EAAT3 NAM, UCPH-42000: ACS Chem Neurosci 2019
- GABAA receptors
- Methaqualone analogs: ACS Chem Neurosci 2020
- Methaqualone analogs: ACS Chem Neurosci 2020
- Mixed targets
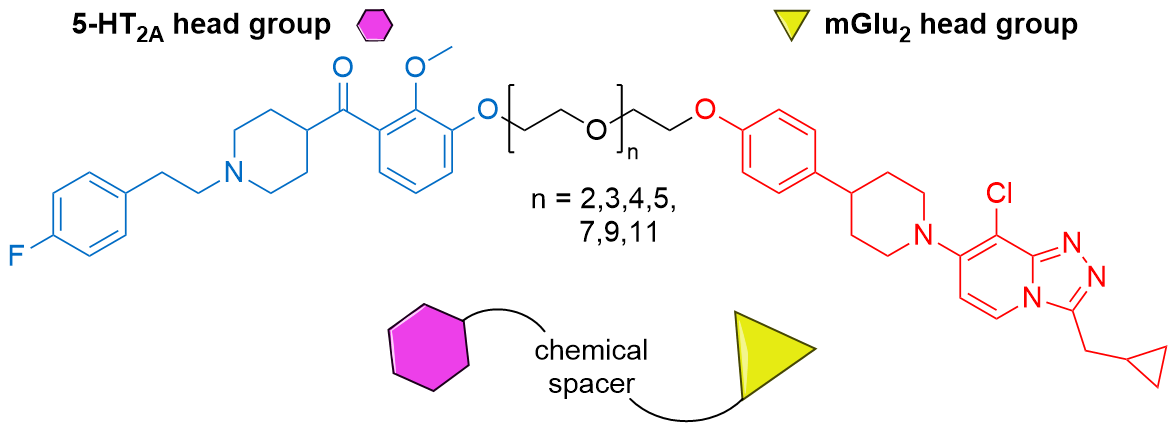
- Putative 5HT2a-mGlu2 receptor complex: J Med Chem 2020
Natural products remain an important source for de-novo drug discovery. Compared to synthetic libraries, natural products comprise a higher ratio of sp3 to sp2 carbon, number of chiral centers and they are in general chemically more complex (number of functional groups pr carbon). We engage in the total synthesis of natural products with the aim of understanding their mode of action and to be able to perform a detailed SAR study.
Most recently we have reported the stereoselective total synthesis of the lactam of the neurotoxin caramboxin in only 8 steps starting from readily available allylglycine:
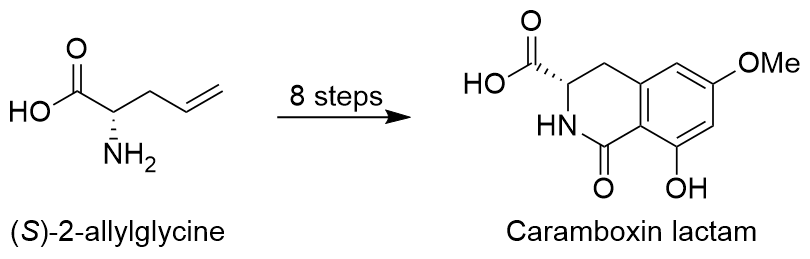
Published in: Chem. Eur. J. 2021, in press
We are committed to discover new synthetic methodology relevant for medicinal chemistry. This be efficient synthesis of chemically complex fragments, new planar heterocycles, new/improved protecting group chemistry and more! Recent and ongoing work is:
Expedite Synthesis of Polysubstituted meta-Halophenols (Chem Eur J 2021)
Phenols are frequent motifs in pharmaceutical agents as well as in agrochemicals. Thus, their halo-functionalized analogues are highly valuable building blocks in the synthesis thereof. We have demonstrated the expedite synthesis of polysubstituted meta-halo phenols by the first-time reported anion-accelerated 2-pi-electrocyclic ring-opening reaction:
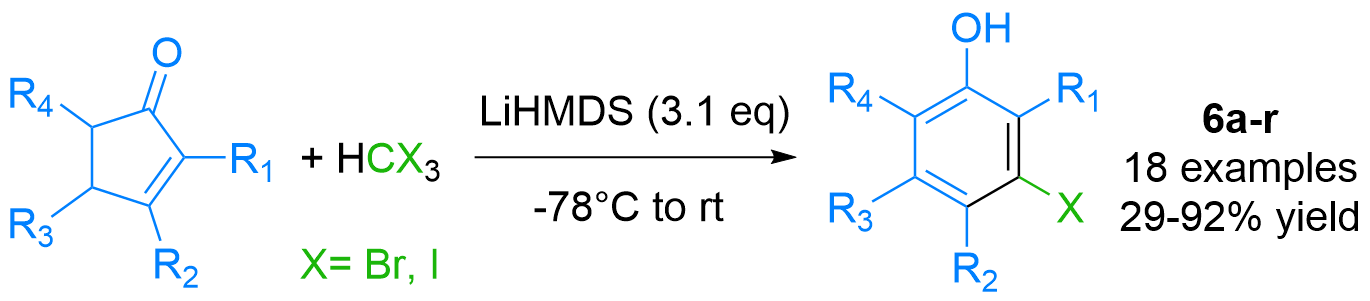
Transition Metal-free Synthesis of Poly-substituted Anilins (Chem Eur J 2022)
The anion-accelerated 2-pi-electrocyclic ring-opening reaction methodology was expanded to the synthesis of anilins - another important motif in medicinal chemistry:

We continue to explore the scope and limitations of this new and exciting methodology!
Novel Planar Heterocycles
Chemical space is enormous! It is estimated to comprise some 10(200) distinct drug-like molecules which every single one displays a unique biological activity profile. We are interested in the development of new methodology for the synthesis of new planar heterocycles as such can be incorporated in novel drug-candidates to induce target selectivity, increase potency and/or fine-tune the bioavailability profile.
We have disclosed the first synthesis of oxazolo[4,5-b]pyrazines by a new palladium-catalyzed domino reaction with an intramolecular ring closure of an N-(2-chloro-3-heteroaryl)arylamide:
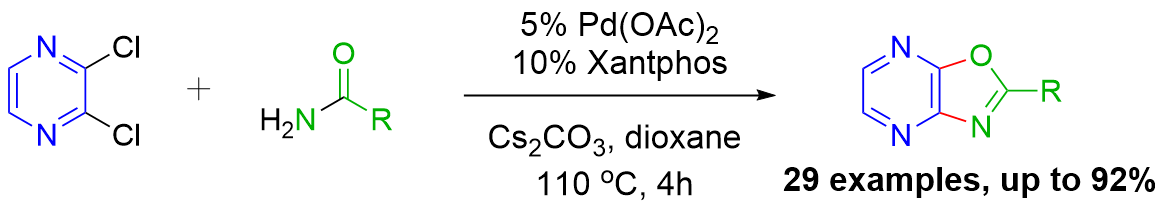
Published in: Adv Synth & Cat 2014
Highlighted in: Synfacts 2014
Methodology for its functionalization was investigated in collaboration with Prof. Florence Mongin, France and published in Eur J Org Chem
--
TBAF Cleaves Boroxazolidones to Corresponding Free Amino Acids
Protection of α-amino acids with 9-borabicyclo[3.3.1]nonane (9-BBN) to give their corresponding boroxazolidones is highly attractive as it concurrently masks both the amino and the carboxylic acid functionalities. However, the required harsh methods for their deprotection have limited its use. In this work we report that tetrabutylammonium fluoride (TBAF) is a mild and versatile reagent for cleaving boroxazolidones to their corresponding free α-amino acids, in high yields.

Published in: Eur J Org Chem 2017
MSc thesis projects
We welcome letter of interests from prospective MSc thesis students who are interested in medicinal chemistry projects and/or organic chemistry projects relevant for medicinal chemistry.
- Letter of motivation (1 page)
- Your CV (1 page)
- List of publications (optional)
Available MSc Thesis projects
All correspondence should be directed to Lennart Bunch.
PhD fellowship positions
We welcome letter of interests from MSc students or MSc graduates who are interested in doing a PhD in organic chemistry or medicinal chemistry. A PhD fellowship can be funded in a number of ways:
- Joint application to a public or private foundation
- Self funded
To learn more about the PhD study program at University of Copenhagen, Faculty of Health and Medical Sciences, click here. Express you interest by sending as PDF:
- Letter of motivation (1 page)
- Your CV (1 page)
- List of publications
All correspondence should be directed to Lennart Bunch.
Postdoc positions
We welcome applicants who are interested in a Postdoc position. Projects of the highest international standard are available within all our research topics. The Postdoc position can be funded in a number of ways:
- Joint application
- Self-funded
If you would like to learn more about how to become a Postdoc in the group, please send as PDF:
- Letter of motivation (1 page)
- Your CV (1 page)
- List of publications
- Plan for raising funding from your own country
All correspondence should be directed to Group leader Lennart Bunch
I established my research group in 2007 and has a strong international profile comprising students and co-workers from several countries. The working environment is vibrant and ambitious, and the language of daily communication is English - both spoken and written.
Supervision
I hold biweekly project meetings with every group member, where we discuss recent progress, challenges, and goals. Aside from this, I run an open door policy everyday after 13:00. You are welcome to drop by.
Outrearch
We engage in scientific and social activities with the other chemistry groups at the department as well as with our collaborating partners.
Goals
Organic chemistry and medicinal chemistry at the highest level. Our research objectives are:
- New synthetic methodology to easily access structurally complex molecules
- New reagents with relevance for medicinal chemistry
- Design and synthesis of novel tool compounds for use by our collaborators in the studying of neurological mechanisms in the healthy and diseased brain
Group members
| Name | Title | Phone | |
|---|---|---|---|
| Christoffer Mentz | PhD Fellow | ||
| Effimia Valavani | PhD Fellow | ||
| Joakim Bøgelund Jakobsen | Postdoc | +4535329013 | |
| Lennart Bunch | Professor | +4535336244 |
| Shengxiao Qi | PhD Student |

