Drug Safety – Sessa Group
Situated at the convergence of applied data science and pharmaceutical science, we leverage large biomedical data and pioneer top-tier Artificial Intelligence solutions and intelligent automation tailored to Pharmacovigilance and Pharmacoepidemiology. With robust industry and regulatory partnerships, we address complex challenges and explore research questions within these domains.

|
The Drug safety group harnesses large and heterogeneous biomedical real-world data, strategically integrating cutting-edge technologies like Artificial Intelligence (AI) and intelligent automation to propel advancements in Pharmacovigilance and Pharmacoepidemiology. Our mission is to revolutionize healthcare by enhancing the safety and effectiveness of treatments. Situated at the crossroads of applied data science and pharmaceutical science, our group is distinguished for fostering true interaction among top-tier researchers in these fields. This collaboration is not just a juxtaposition of expertise but a dynamic fusion that propels innovation. |
An important strength of our groups lies in our close ties with industry and regulatory agencies partners. Their invaluable input creates exciting opportunities to seamlessly apply our innovative research in real-world scenarios.
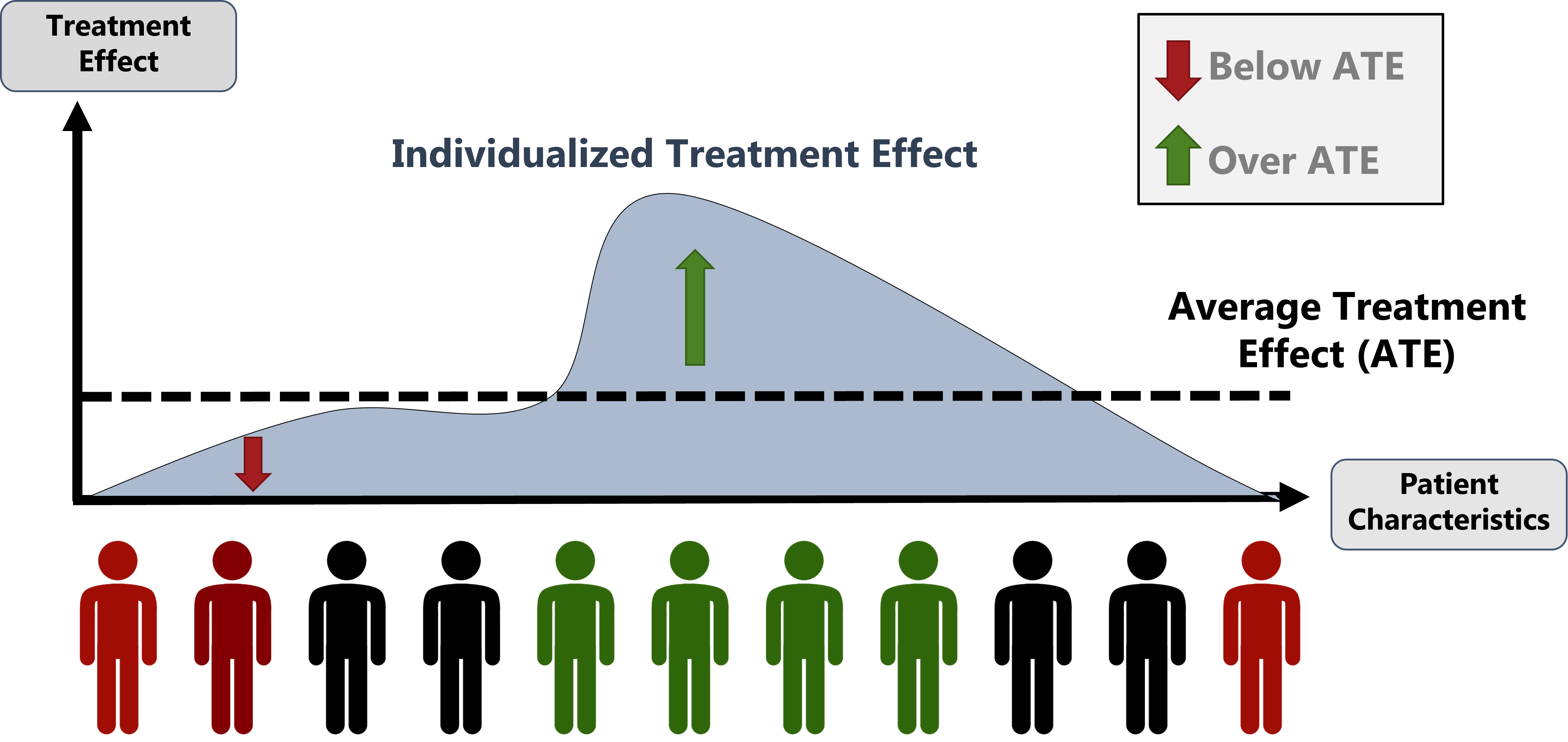 Figure explaining the importance of estimating individualized treatment effects when providing precision medicine recommendations rather than average treatment effects.
Figure explaining the importance of estimating individualized treatment effects when providing precision medicine recommendations rather than average treatment effects.
Precision Medicine Revolution: Integrating Big Data with AI
We are pioneering precision medicine by integrating various omics data with a diverse range of datasets. Leveraging electronic health records (EHRs), administrative and healthcare registers, spontaneous reporting system (SRS) databases, and biochemical and immunological biomarkers, our innovative machine learning (ML) techniques are enhancing the characterization of individual patients' risks—whether it be adverse drug reactions (ADRs), adverse events (AEs) following immunization, or lack of therapeutic efficacy.
Revolutionizing Patient Care: Personalized Risk Assessment
By integrating omics data and advanced ML, we are elevating diagnostic accuracy, accounting for co-morbidities, and reliably predicting patient trajectories. This transformative strategy aims to optimize targeted treatment interventions, ensuring that each patient receives the most effective and personalized care.
Precision Medicine in Action: Personalized "Counterfactuals"
An essential element of precision medicine involves the creation of personalized "counterfactuals" using causal ML. They provide valuable insights into potential outcomes if a patient decides to delay treatment, opts for an alternative approach, or chooses no treatment at all. By exploring these personalized scenarios, patients and their healthcare providers gain a nuanced understanding of the potential consequences of significant decisions before they are made. In our group we are conducting extensive research on the use of ML for Personalized "Counterfactuals".
Forecasting Health Outcomes: The Power of Machine Learning
We navigate and research complexities such as estimating lengths of stay, predicting complexities across different units (e.g., ward, high-dependency unit, intensive care), understanding responses to resuscitation efforts, forecasting the likelihood of readmission, and comprehending the outcomes associated with readmission. This forward-thinking approach ensures a comprehensive understanding of patient journeys and enables proactive, personalized healthcare.
Strength in Collaboration: The Core of Precision Medicine
The successful implementation of precision medicine hinges on access to relevant data, up-to-date knowledge in ML, and robust partnerships with healthcare providers, industry, and regulators. These elements are the pillars of strength for the Drug Safety Group.
Examples of projects
Here’s a good example of pioneering work – led by the Group leader, Associate Professor Maurizio Sessa in a project financed by the prime ministers of the Nordic Countries – on developing an AI-enabled precision medicine tool to predict individuals at higher risk of COVID-19 hospitalization and mortality.
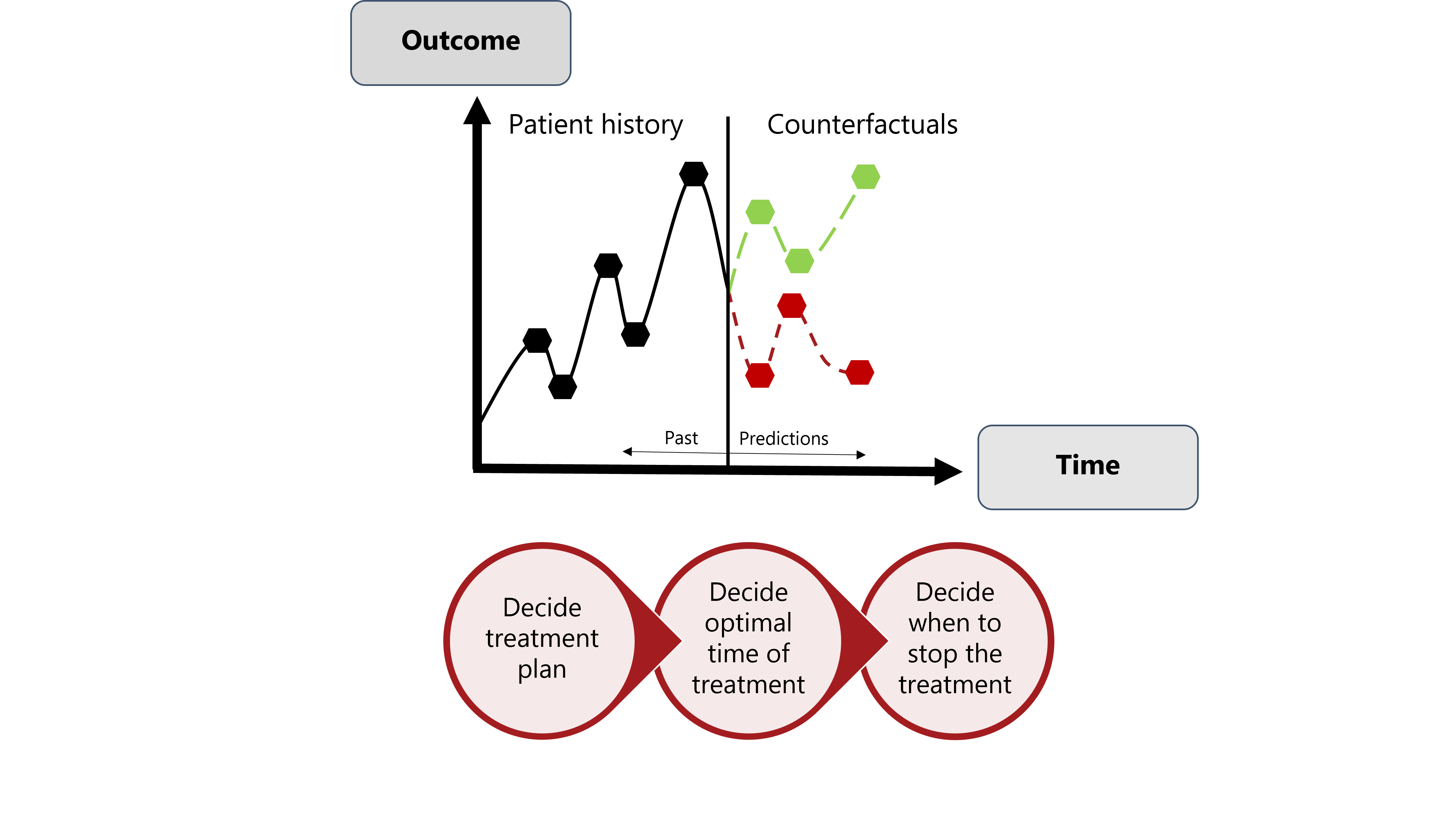 Figure explaining the importance of estimating controfactual outcomes when providing precision prevention recommendations.
Figure explaining the importance of estimating controfactual outcomes when providing precision prevention recommendations.
Proactive Healthcare with AI Innovation
The Drug Safety Group is dedicated to the invaluable task of identifying potential health issues in large populations even before symptoms manifest. Our mission revolves around the research and development of technology that enables cost-effective, early, and efficient interventions, bringing significant benefits not only to public health but also generating savings within the healthcare economy.
Empowering Proactive Health: Early Detection with AI Precision
Our primary goal is to pioneer technology capable of identifying groups of individuals in a pre-symptomatic stage, enabling us to reliably predict the development of common chronic conditions. This ambitious endeavor involves harnessing the power of AI to analyze extensive primary care and community clinical datasets. We integrate genomics and social demographics datasets, including deprivation indices and ethnicities, along with indicators of patient engagement in self-healthcare management, also known as patient activation.
Holistic Approach: AI-Driven Insights
Our approach is holistic, utilizing AI to delve into diverse datasets for a comprehensive understanding. By integrating genomics, demographics, and patient engagement data, we research how to create sophisticated AI-driven models that identifies potential health risks with unparalleled precision, facilitating proactive and targeted interventions.
Examples of projects
Here’s a good example of pioneering work – led by the Group leader, Associate Professor Maurizio Sessa in a project financed by the prime ministers of the Nordic Countries – on developing an AI-enabled precision medicine tool to predict individuals at higher risk of pneumonia hospitalization.
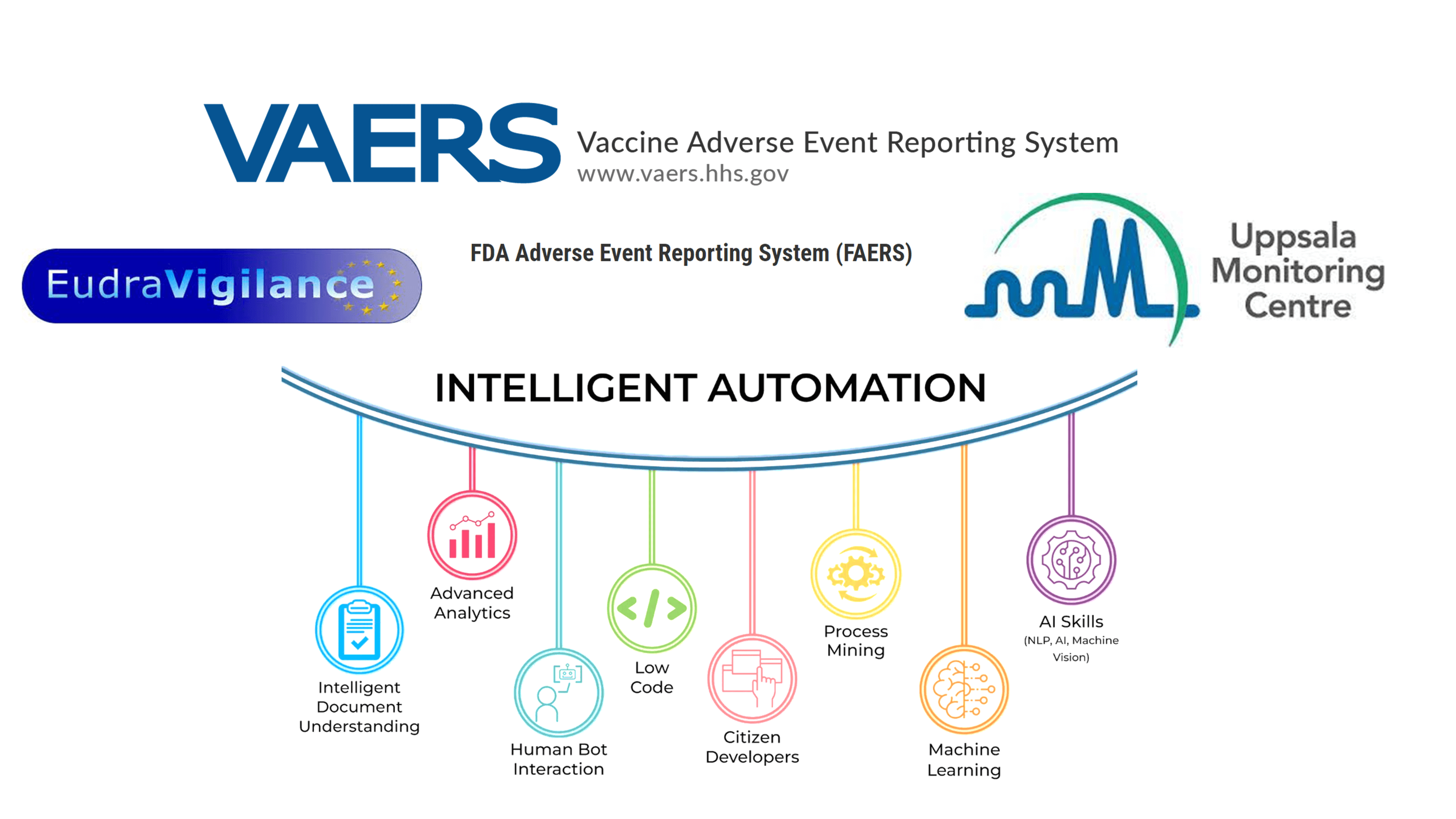 Figure explaining the possible areas of implementation of intelligent automation in pharmacovigilance activities based on spontaneous reporting databases.
Figure explaining the possible areas of implementation of intelligent automation in pharmacovigilance activities based on spontaneous reporting databases.
Advanced Pharmacovigilance with AI
Our mission in the Drug Safety Group is to propel pharmacovigilance into the future by leading the research and application of AI to automate intricate and labor-intensive processes.
Automating Causality Assessment with AI Precision
A primary area of emphasis is the causality assessment. Here, we researh how to harness the power of AI to systematically evaluate and determine the likelihood of a causal relationship between a drug or a vaccine and an adverse event (AE). Recognizing the complexity and time-consuming nature of this process, our innovative use of AI aims to streamline and enhance the accuracy of these critical assessments.
Unraveling Complexities: Identifying Alternative Causative Agents
Addressing the challenge of identifying alternative causative agents in drug-event pairs, we research how to employ AI to investigate potential factors beyond the primary drug that may contribute to AEs. This forward-thinking approach allows us to efficiently analyze vast datasets, unveiling patterns and associations that might escape notice through traditional methods.
Signal Detection and Validation: Proactive Safety Measures
Central to our focus is signal detection and validation. With AI, we research how to navigate extensive databases like EudraVigilance, VigiBASE, FAERS, VAERS, and more, unveiling signals or patterns that may indicate previously unrecognized risks or safety concerns associated with specific drugs and vaccines. This proactive stance enables timely responses to emerging safety issues.
Prioritizing Safety: Biological and Temporal Plausibility
Our efforts extend to research how to prioritize signals based on their biological and temporal plausibility. AI algorithms play a crucial role in evaluating the significance of signals, considering factors such as the biological mechanism underlying an observed effect and the temporal relationship between drug exposure and AEs. This prioritization ensures resources and attention are directed toward the most critical and time-sensitive safety concerns.
Leadership in AI-Driven Pharmacovigilance: A Comprehensive Approach
In summary, our group leads the charge in researching and applying AI to pharmacovigilance, employing a comprehensive approach to automate critical tasks. Through pioneering techniques and exploration of several SRS databases, our aim is to enhance the efficiency, accuracy, and responsiveness of pharmacovigilance processes. Join us in our commitment to continuous improvement in drug safety and patient care.
Examples of projects
Here’s a good example of pioneering work – led by the Group leader, Associate Professor Maurizio Sessa in a project aimed at developing an AI-enabled signal detection system using advanced machine learning techniques in FAERS.
Here’s a good example of pioneering work – led by the Group leader, Associate Professor Maurizio Sessa in a project aimed at developing an AI-enabled signal detection system using natural language processing in VAERS.
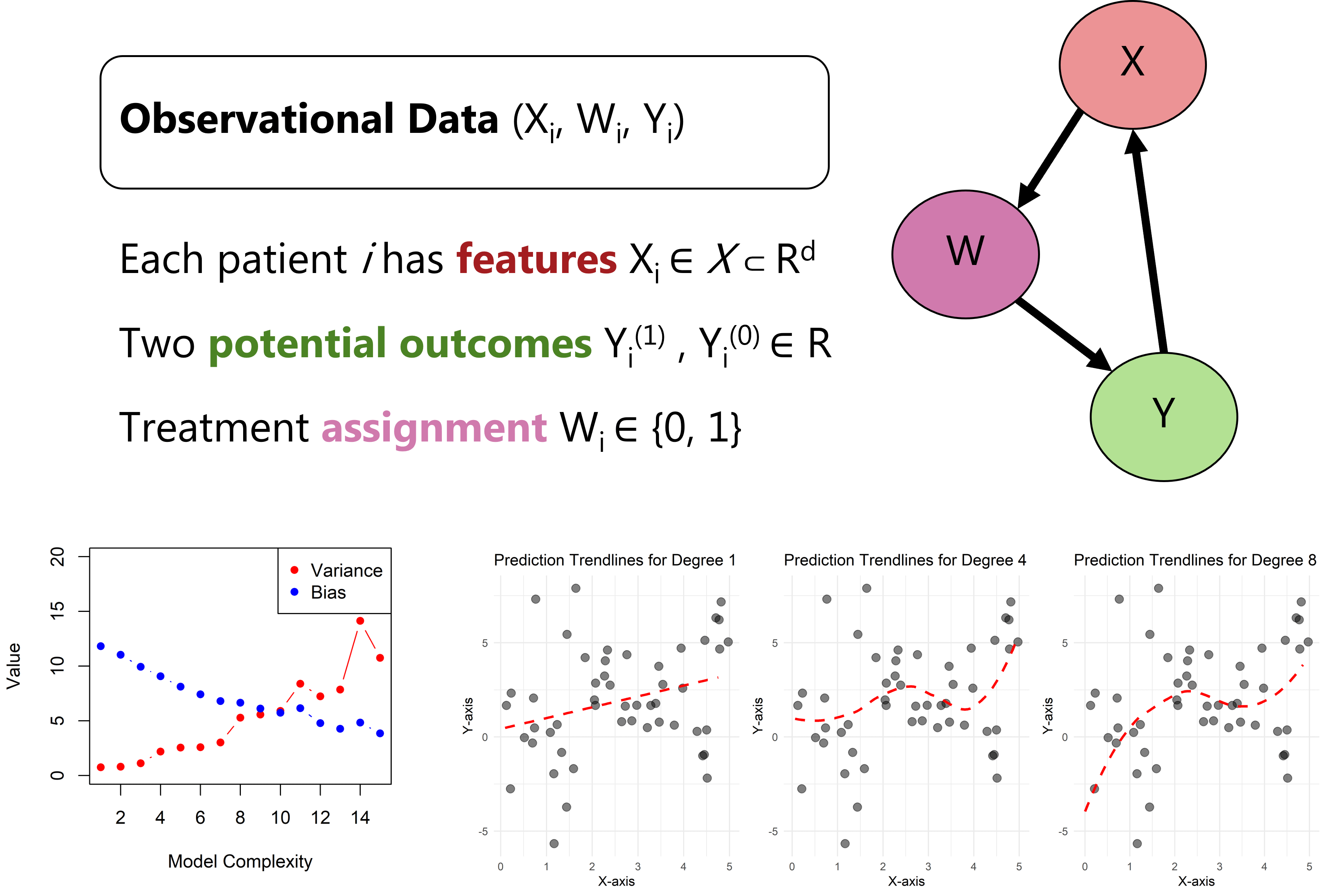 Figure explaining the causal machine learning implementations in observation data.
Figure explaining the causal machine learning implementations in observation data.
Transforming Pharmacoepidemiology through AI
We are committed to shaping the future of pharmacoepidemiology through the research of strategic implementation and appliactions of AI. Our mission revolves also around the research of intelligent automation of labor-intensive processes, pushing the boundaries of what's possible in healthcare research.
At the core of our mission is the strategic research on the application of AI for the intelligent automation of labor-intensive processes in pharmacoepidemiology. We are actively involved in reearching and developing cutting-edge AI-driven algorithms designed to assess medication use, employing causal ML techniques for a nuanced understanding of heterogeneous treatment effects, and optimizing prediction and estimation functions for enhanced accuracy.
Driving Innovation with Data: Nordic Countries and Beyond
Our commitment to excellence extends to our data-driven approach. Leveraging big data, we draw insights from Nordic countries' administrative and healthcare databases, as well as registers from other nations. Our research is anchored in a dynamic environment at the crossroads of academia, healthcare, authorities, and the pharmaceutical industry.
Collaboration and Networks: Building a Global Impact
Collaboration is at the heart of our success. We work closely with national and international research groups, proudly contributing to the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP) and the Nordic Network for Pharmacoepidemiology (NorPEN). Together, we are shaping the future of pharmacoepidemiological research.
Leaders in Education and Training: Empowering the Next Generation
Beyond our research initiatives, we take pride in being a leading provider of education and training in AI-driven pharmacoepidemiology. By imparting knowledge and skills, we aim to empower the next generation of researchers and practitioners in our dynamic field.
Examples of projects
Here’s a good example of pioneering work – led by the Group leader, Associate Professor Maurizio Sessa in a project aimed at developing an AI-enabled detection of drug exposure in administrative and healthcare registers.
Machine learning-driven development of a disease risk score for COVID-19 hospitalization and mortality: a Swedish and Norwegian register-based study.
Front Public Health. 2023 - DOI: 10.3389/fpubh.2023.1258840
Optimizing Signal Management in a Vaccine Adverse Event Reporting System: A Proof-of-Concept with COVID-19 Vaccines Using Signs, Symptoms, and Natural Language Processing.
Drug Saf. 2023. - DOI: 10.1007/s40264-023-01381-6
AI-based disease risk score for community-acquired pneumonia hospitalization.
iScience. 2023 - DOI: 10.1016/j.isci.2023.107027
Developing an Artificial Intelligence-Guided Signal Detection in the Food and Drug Administration Adverse Event Reporting System (FAERS): A Proof-of-Concept Study Using Galcanezumab and Simulated Data.
Drug Saf. 2023 - DOI: 10.1007/s40264-023-01317-0
A novel approach for pharmacological substantiation of safety signals using plasma concentrations of medication and administrative/healthcare databases: A case study using Danish registries for an FDA warning on lamotrigine.
Pharmacol Res. 2023 - DOI: 10.1016/j.phrs.2023.106811
A machine-learning guided method for predicting add-on and switch in secondary data sources: A case study on anti-seizure medications in Danish registries.
Front Pharmacol. 2022 - DOI: 10.1016/j.phrs.2023.106811
Artificial Neural Network vs. Pharmacometric Model for Population Prediction of Plasma Concentration in Real-World Data: A Case Study on Valproic Acid.
Clin Pharmacol Ther. 2022 - DOI: 10.1002/cpt.2577
Pharmacological and epidemiological considerations while constructing treatment episodes using observational data: A simulation study.
Pharmacoepidemiol Drug Saf. 2022 - DOI: 10.1002/pds.5366
PhD Students/Postdocs
We are always interested in hearing from highly motivated candidates in both pharmacovigilance, pharmacoepidemiology and/or applied data science. Please send us a letter of intent outlining your motivation to join, as well as your publication list and CV.
Potential candidates who are interested in joining should be competitive to apply for external fellowships and sources of funding such as EMBO Long-Term Fellowship, Marie-Curie Fellowship, HFSP Fellowship or other application options for postdocs.
When available, fully funded postdoctoral fellowships will be posted here. Please apply using the online system, as e-mailed applications will not be considered.
MSc/Exchange/Scholarship students
We are happy to receive inquiries from pharmacy/pharmaceutical science students and/or students interested in pharmacovigilance/pharmacoepidemiology or applied data science projects. Master students who are interested in experimental projects for their final MSc thesis are typically staying for 6 - 12 months.
Researchers
| Name | Title | Phone |
|---|
Other members:
| Name | Title | Phone | |
|---|---|---|---|
| Lampros Spiliopoulos | PhD Student |

