BioSAXS
One prime focus in our group is structural analysis of protein fibrillation.
We analyse fibrillating proteins during the fibrillation process, in a time-resolved manner. This has enabled us to present the first ever solution structure of a structural fibrillation nucleus (Vestergaard, Groenning et al. 2008), based on data from maturing insulin fibrils. On-going projects include analysis of Parkinson's-related alpha-synuclein, human transthyretin (associated with senile systemic amyloidosis), the Alzheimers related amyloid-beta, glucagon-like peptide 2, and selected model peptides from fibrillation-prone proteins.
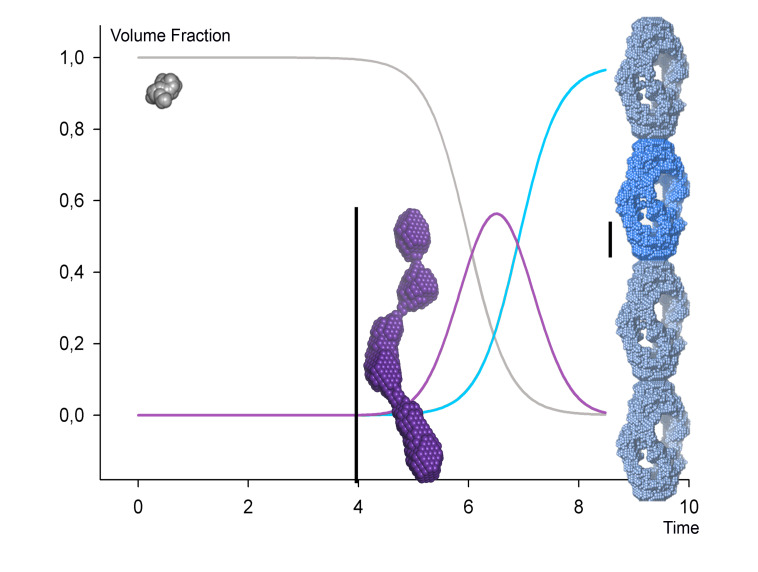
The insulin monomer is to scale with the oligomer, and a 200 Å length scale is shown next to the insulin oligomer and fibril repeating unit respectively. Note that only one repeating unit of the fibril is modelled from the SAXS data (shown in more intense blue color). The intertwining fibril has been modelled by simple translation of individual repeats. Modified from (Langkilde & Vestergaard (2012)).
Vision
The vision of our group, is to establish a full structural understanding of protein fibrillation.
In our group, we investigate a number of pharmaceutically relevant protein systems. We apply biophysical and structural methods for macromolecular characterisation, with prime expertise in solution small angle X-ray scattering (SAXS). SAXS has the advantage that structural analysis can be performed on macromolecules directly in solution. The method is hence highly complementary to macromolecular crystallography. Working in solution has the advantage that mixtures, complex formation, structural changes and flexible systems may be investigated. In our group we use SAXS for all the mentioned types of analysis.
We use synchroton X-ray data in our studies, and greatly acknowledge both the frequent access to as well as the great support from the skilled staff at the international large-scale facilities.
We involve with topic driven SAXS method development. One example is the bioXTAS project (www.bioxtas.org); which although at present not funded, still results in collaborational efforts at SAXS beamlines. Another example is model-free decomposition of data from complex evolving protein systems, as exemplified in (Giehm et al. (2011)). We are currently working on improving and partially automating this type of data analysis.
The group thankfully acknowledges generous funding from:
- The Danish Council for Independent Research – Medical Sciences (Sapere Aude programme)
- The Carlsberg Foundation
- The Drug Research Academy
- Novo Nordisk A/S
- DANSCATT
- FP7 – People (Marie Curie)
- The University of Copenhagen, Faculty of Health and Medical Sciences
Group Leader
Annette Eva Langkilde
Associate Professor
Phone +45 3533 6202
annette.langkilde@sund.ku.dk>
Group/project members
| Name | Title | Phone | |
|---|---|---|---|
| Langkilde, Annette Eva | Associate Professor | +4535336202 |

