Molecular Disease Biology
These cell lines were characterized by thorough omics, which includes exome sequencing, SNP gene copy number analyses, two different types of methylation profiling, microRNA, RNA sequencing, micro array gene expression and proteomics.
Based on data from this research platform we have contributed to two ongoing clinical trials in which breast and CRC patients are analyzed for the level of a predictive biomarker and – if positive – offered a treatment they would otherwise not receive (repurposing of drugs).
Below, four selected examples highlight the translational potential of such a research platform.
- Biomarker research
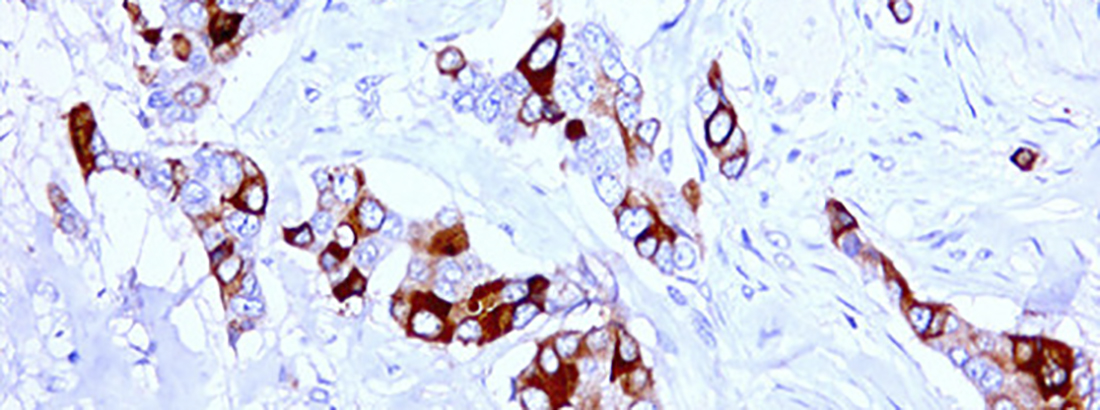
In SN-38 resistant colorectal cancer (CRC) cells we found that a major resistance mechanism is up-regulation of the drug efflux pump ABCG2. A negative predictive value of high ABCG2 expression for response to irinotecan was verified in clinical material from the PETACC-3 randomized clinical trial and in a Danish cohort. - Repurposing of non-anti-cancer drugs
The major advantages of repurposing drugs that are already on the market is that they can be tested directly in a clinical phase II study since they have already passed phase 1 for their original medical indication. In our resistant CRC cells, we have shown an additive/synergistic effect of disulfiram (Antabuse) ± copper with oxaliplatin in SN-38 resistant cell lines and with SN-38 in oxaliplatin resistant cell lines. Based on prior clinical trials and our cell line data we are currently planning a phase II clinical trial enrolling mCRC patients relapsing from 1st line treatment with either oxaliplatin or irinotecan containing treatment. - Test of novel chemical entities for anti-cancer effects
We searched for compounds that could be active in CRC patients who develop resistance to the topoisomerase-I (Top1) inhibitor irinotecan (SN-38). We found that the Top1 inhibitor LMP-400 was active in our SN-38 resistant cell lines, likely because LMP-400 is not pumped out of cells by ABCG2. LMP-400 is currently in early clinical trials. - Translational research: Clinical trials based on a drug resistant cell line research platform
New treatments are needed for metastatic CRC patients, as the objective response rate to 3rd line treatment is approximately 5%. Our group found that 10% of CRC patients harbor an amplification of the TOP2A gene, encoding the Top2A enzyme which is targeted by anthracyclines. TOP2A amplification predicts improved efficacy to epirubicin (an antracycline) in breast cancer. So, epirubicin could be an alternative to irinotecan-based therapy in CRC patients with TOP2A amplification who relapsed on oxaliplatin containing chemotherapy. In our resistant cells we showed lack of cross-resistance to epirubicin in 2 of 3 oxaliplatin resistant cell lines but full cross resistance in SN-38 resistant cell lines. This lead to initiation of a phase II prospective clinical trial in which metastatic CRC patients with TOP2A amplification are offered epirubicin as 2nd line treatment instead of irinotecan. The very first patient in the protocol obtained a significant confirmed partial response (PR) according to the RECIST 1.1 criteria.
Resistance to anti-cancer drugs is a major clinical problem eventually leading to deaths of cancer patients. We have establishes a research platform consisting of drug sensitive and drug resistant cancer cells. Based on this research platform and analyses of clinically relevant tissue we are conducting research within biomarkers, repurposing of non-anti-cancer drugs, test of novel chemical entities for anti-cancer effects and translational research (Clinical trials based on a drug resistant cell line research platform).
- Danmarks Grundforskningsfond

- Breast Cancer Research

- NEYE-Fonden
Group members
| Name | Title | Phone |
|---|