Cancer Precision Medicine in the Moreira Group
The Precision Cancer Medicine Group strives to identify cancer molecular biomarkers, applying omics technologies to maximize molecular insight into individual tumours. Patient tissue analysis complemented by model systems, animal as well as cellular, are used to accelerate drug development and enable individualized disease treatment and prevention.
The cancer burden is expected to increase in Europe from 4.4 million cases in 2020 to 5.3 million cases in 2040; an increase of approximately 21%. This anticipated trend is due partly to population aging and growth, but also to lifestyle changes with increasing prevalence of risk factors, such as tobacco, obesity, diet and physical inactivity.
Despite improvements in diagnostic techniques and introduction of targeted treatments, a substantial number of cancer patients will eventually die from their disease. One of the main reasons for this dismal outlook is that cancer cells have an inherently high mutational rate, which drives them to acquire a more aggressive behaviour and develop resistance to anti-cancer drugs. As a result, all successful cancer therapies are limited by the development of drug resistance. To date, the way to address this problem has been to develop novel, more effective therapies, based on our increasing knowledge of cancer biology. But this is essentially a one-size-fits-all approach that does not consider individual variability in genes, environment, and lifestyle for each person.
Our group uses a combination of cellular models with molecular analysis of clinical samples to identify biomarkers and novel therapeutic targets that can be matched to individual patients and may improve clinical management for each cancer patient.
Research Projects
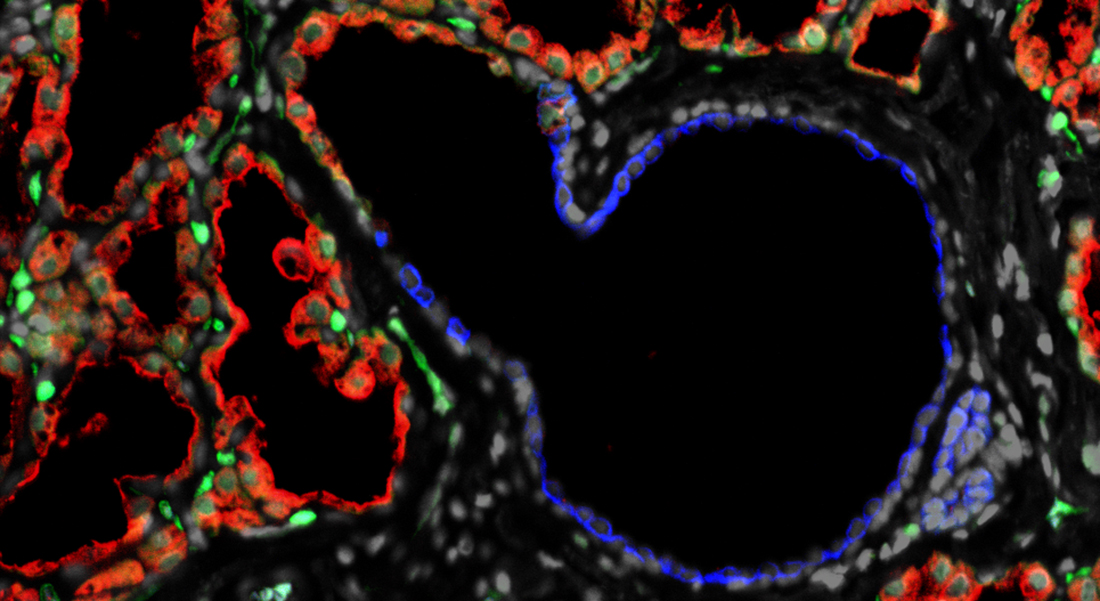
One cancer whose incidence is projected to increase dramatically is colorectal cancer (CRC). CRC is a life-threatening disease with high incidence, morbidity, and mortality, and constitutes a significant societal burden. In Europe, the number of new CRC cases is anticipated to rise by about 53%, from 1.88 million cases in 2020 to 2.87 million by year 2040. This dramatic increase is due to increased commonness of Western lifestyle-linked factors, such as high-fat diets, processed meat consumption, obesity, alcohol use, and physical inactivity, all of which have been associated with colorectal cancer. In the case of CRC, there is strong evidence that an early diagnosis leads to a better prognosis, with metastatic CRC having a 5-year survival that is only slightly greater than 10%, compared with up to 90% for stage I CRC. Clearly then, biomarkers for early detection of CRC would have a major clinical impact.
Our group is investigating coherent processes for biomarker discovery in CRC that could increase the probability of identifying biomarkers of clinical value.
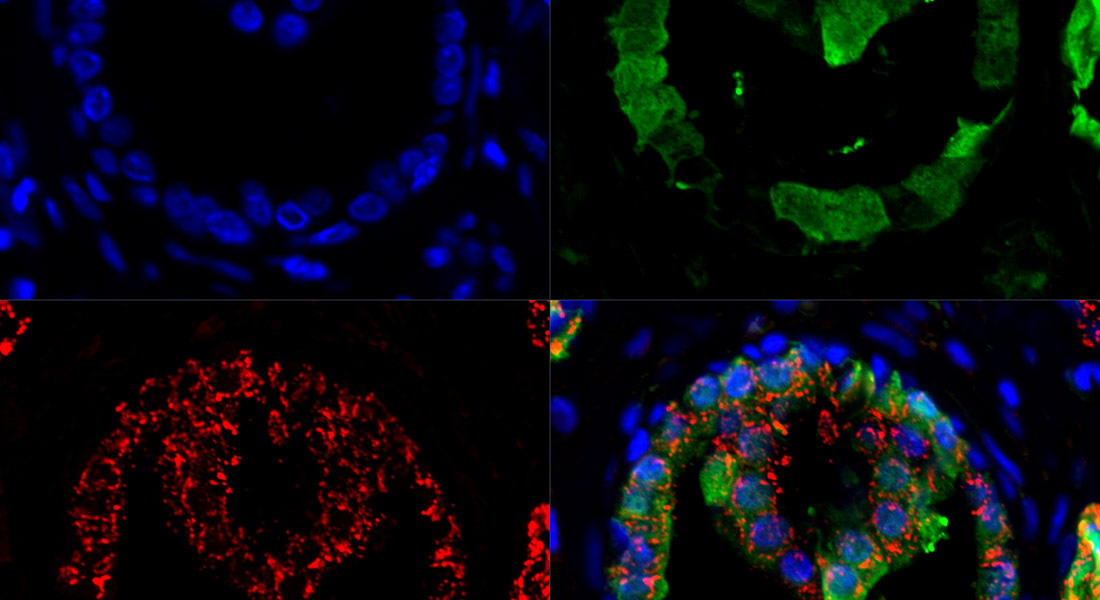
Adenomas are benign neoplastic lesions, but there is strong evidence that some of these lesions can develop into invasive carcinomas even after surgical removal. At present we know very little on how to risk-stratify these early neoplastic lesions, and as a result all adenoma patients are intensely surveyed, undergoing frequent time-consuming and expensive and invasive examinations.
Our group works to identify novel biomarkers in adenomas that are predictive of later colorectal cancer development with the hope of being able to identify those adenoma patients who should be offered increased surveillance and/or medical intervention therapy.

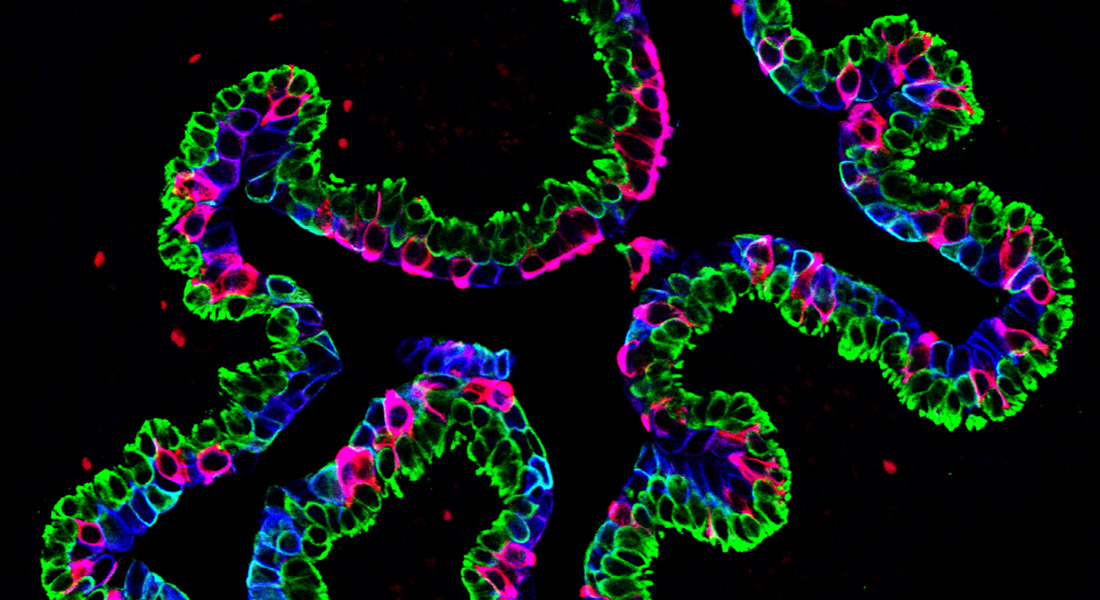
Current strategies for reducing colorectal cancer incidence and mortality are mainly based on secondary prevention initiatives, such as screening programs, with focus on early detection and removal of precancerous lesions. But primary prevention strategies based on the use of pharmacologic agents offer a promising approach to control cancer. Over the past three decades, a growing body of literature has emerged, based on multiple lines of evidence -from cell based data to observational epidemiological studies- suggesting aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs), can suppress carcinogenesis and delay development of cancer. Aspirin (acetylsalicylic acid) is a commonly prescribed NSAID, with antithrombotic and anti-inflammatory actions, which has raised considerable attention as a potential chemopreventive agent. Salicylate, the primary metabolite of aspirin, irreversibly acetylates and inactivates cyclooxygenase (COX) enzymes, inhibiting prostaglandin synthesis. But salicylate modulates multiple additional molecular targets, many of which may also contribute to its anticancer effect in CRC. Although multiple COX-dependent, as well as -independent mechanisms, have been proposed, the precise molecular events underpinning the anticancer effects of aspirin are yet to be unequivocally established.
Our group conducts experiments designed to elucidate the molecular mechanism(s) underlying the cancer prevention effect of aspirin, with the aim of discovering a pharmacological alternative.

Prostate cancer (PCa) is the most common cancer among men in developed countries, and a major cause of male mortality, mostly due to disease progression to metastatic castration-resistant prostate cancer (mCRPC). Although several chemotherapeutic drugs have been tested in mCRPC, the first one to have shown significant benefit in terms of overall survival was docetaxel, a microtubule-stabilizing taxane. The survival benefit demonstrated in mCRPC was short but significant, and docetaxel is still frequently used as standard first-line treatment of mCRPC. Unfortunately, responses are invariably limited in time and patients eventually succumb to disease progression because of acquired drug resistance.
Our group developed cellular models of resistance to docetaxel and is using these models to identify biomarkers of resistance and to discover new drugs that can revert or bypass drug-resistance.
Group/project members
| Name | Title | Phone |
|---|

