Pharmacometrics
Describing the plasma concentration-time profile (pharmacokinetics, PK) and understanding the quantitative link to therapeutic response (pharmacodynamics, PD) of a drug is paramount for
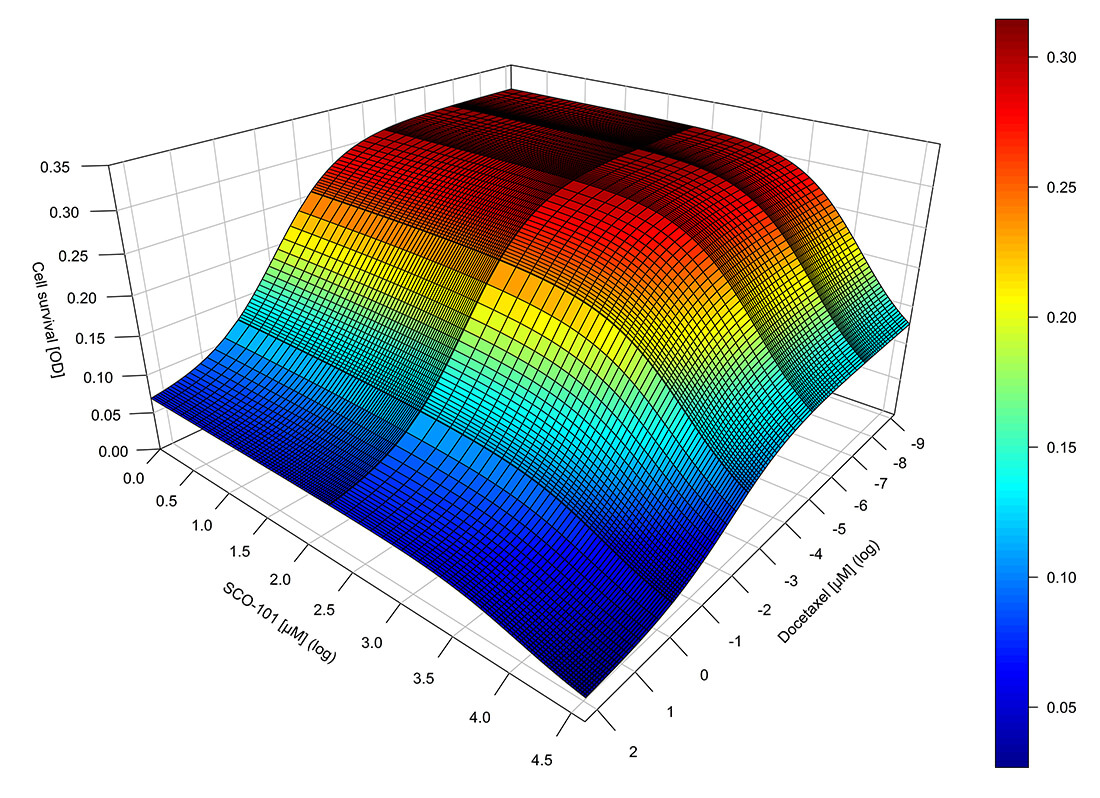
PKPD modelling (also referred to as pharmacometrics or quantitative pharmacology) is the science of developing computer-based mathematical and statistical models that provide useful mechanistic understanding of the processes involved in drug exposure and corresponding therapeutic effect as a function of time.
Non-linear mixed effects - or population PKPD - modelling allows for analysis of both the population mean response in addition to description and explanation of inter-individual differences in therapeutic response and characterization of residual variability in studies.
Population PKPD models can be used for simulation of study trial outcome with different dosage regiments. Furthermore, PK-PD models based on translational biomarkers is a useful tool to predict patient response based on experimental disease models. This makes PK-PD modelling a strong analytical tool that plays an increasing role in drug research & development in the pharmaceutical industry.
PKPD modelling is applicable to a wide range of pharmacological areas and can be used to analyze data from both animal and human studies.
Examples of projects we are working on include:
- Population PKPD modelling of the analgesic response of opioids in post-operative pain
- Personalized medicine with desmopressin: dose individualization in the treatment of central diabetes insipidus and bleeding disorders
- Fixed dose combinations in drug development - Design of trials for identifying optimal combination ratios using advanced computer modelling tools
- The development of fixed-dose combinations (FDCs) through improved methodology – seen from a regulatory perspective
- Differences in pharmacokinetic parameter values in obese and normal weight adolescents
We are using population PKPD modelling programs such as NONMEM with Pirana, PsN, Xpose and R.
We currently have international scientific collaborations with highly esteemed academic pharmacometrics research groups and clinical research units at:
- International academic collaborations:
Pharmacometrics Group, Uppsala University, Sweden
Australian Centre for Pharmacometrics at University of South Australia, Adelaide, Australia - Danish Hospitals
Neuroscience Center, Rigshospitalet, Copenhagen University Hospitals, Denmark
Department of Clinical Pharmacology, Bispebjerg Hospital, Copenhagen, Denmark
Aleris-Hamlet, Ringsted, Denmark - Pharmaceutical Companies:
Ferring Pharmaceuticals, Copenhagen, Denmark
NovoNordisk, Søborg and Måløv, Denmark
Lundbeck A/S, Valby, Denmark - Small Bio-Tech Companies:
Contera Pharma, Copenhagen, Denmark - University of Copenhagen:
Bioanalytical Chemistry and Metabolomics, University of Copenhagen, Denmark
Centre of Regulatory Sciences, Social and Clinical Pharmacy, University of Copenhagen
Biostatistical Department, University of Copenhagen
Group members
| Name | Title | Phone | |
|---|---|---|---|
| Tianwu Yang | PhD Fellow | +4535332844 | |
| Trine Meldgaard Lund | Associate Professor | +4535336340 |
| Srishti Nagaraj | External PhD Student |

