Kohlmeier Group
Levels of arousal underlie whether an organism is awake or asleep and in some sleep disorders, can be regulated improperly. Inappropriate levels of arousal are also associated with drug addiction behaviours.
Our laboratory is interested in elucidating the neurobiology underlying arousal with the hope that we can identify targets for managing or restoring appropriate and adaptive levels of arousal associated with human behaviours.
Accordingly, work in our laboratory is conducted to elucidate the neuroactive chemicals involved in mediation of sleep and the level of arousal. Moreover, we investigate changes in sleep patterning associated with other arousal disorders, such as depression and stress. In addition, we examine the actions pharmacologic agents have on activity levels of neurons importantly involved in generation of arousal state to gain understanding of the mechanisms of action of clinically relevant drugs.

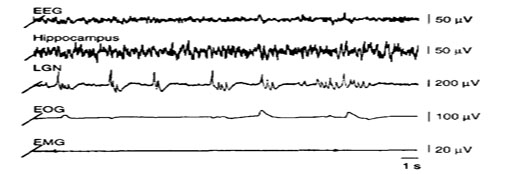
Why study brain arousal?
Arousal – or wakefulness – is important in regulating consciousness, attention, and information processing. Appropriate levels of arousal are crucial for life-sustaining behaviours such as acquisition of food, attraction of mates, learning, and memory. Abnormal arousal levels are part of drug-seeking behaviours, aberrant stress responses, epilepsy, and mental disorders. Hence, pharmacological arousal intervention is part of stress control, treatment of epilepsy and sleep disorders such as narcolepsy and insomnia, and alteration of consciousness (anaesthesia).
Our goal is to characterise the detailed physiological and pharmacological mechanisms responsible for regulating the normal and pathological states of arousal. We aim to:
identify therapeutic targets for restoring appropriate and adaptive levels of arousal
evaluate the effects and side-effects of new and existing drugs on the regulation of normal arousal.
Our approach
Many therapeutic drugs affect arousal levels either by design or as a side-effect. Moreover, the arousal level (stress, pain, addiction) can modulate pharmacological efficacy. Understanding how arousal levels are regulated is therefore of critical importance for rational drug development. Arousal regulation occurs at the level of individual signal molecules, receptors, ion channels and transporters; however, the outcome of arousal regulation is manifested at the systems level as modulation of behavioural states and circuitry output. Hence, a cogent research programme in areas of arousal regulation should include a comprehensive approach at multiple levels of function. Accordingly, we seek to provide a comprehensive elucidation of the mechanistic basis of different types of arousal states at all levels of integration, from the molecule to unanaesthetised animal models.
Our group brings together researchers with expertise in many fields including neuropharmacology, electrophysiology, ethology, surgery, animal models, cell culture, and pharmacodynamics. Our research experience spans studies of single ion channels and receptors to the freely behaving animal. Current and past research focuses include regulation of the organismal states of Addiction, Epilepsy, Stress and Sleep/Wakefulness.
We also perform detailed molecular pharmacological characterisation of ligands with affinity for GABAA and GABAC receptors, most of which have been designed, synthesised and screened by members of the research area Medicinal Chemistry. Through this activity, we have access to a number of interesting ligands to be tested in our models for epilepsy as well as the other arousal pathologies.
Beyond the implications for drug development within the respective specific fields, findings emanating from these projects have important implications for development and improvement of efficacious anesthetic agents, psychotropics, OTC sleeping aids, etc.
- Lundbeck
- Glostrup Hospital
- Hvidovre Hospital
- Department of Neuroscience and Pharmacology
- Department of Biomedical Sciences
- Lab. of Molecular Pathology (SUND),
- Aarhus University
- University of California at San Diego
- New York Medical College
- University of British Columbia
- Electrophysiology (cultured cells, brain slices, in vivo single and multi-neuron recording)
- Calcium imaging of individual networked neurons
- Animal models of pathological states (epilepsy, stress, addiction, narcolepsy/cataplexy, schizophrenia)
- Behavioural testing
- In vivo functional neuropharmacology
- Primary cell culture
- Kinetic modelling of receptor and ion channel function
- Ultra-fast drug application to isolated cells and membrane patches
- Juxtacellular drug application (laser uncaging, microiontophoresis)
- In situ electrophysiological and post hoc immunohistochemical cell identification
Our research projects
Individuals who are addicted to drugs exhibit highly motivated drug-seeking behaviour. This is a highly aroused state in which the focus of the addict is entirely directed towards acquisition of their drug of choice. One strategy to combat addiction is to decrease the prioritised motivational value of the drug by targeting levels of arousal. Drug addiction is a disease of the brain, not a personal choice, which highlights the need for further research to define novel targets to combat this devastating mental condition. Our group conducts experiments designed to elucidate the neurobiology underlying excitation of neural areas involved in arousal by drugs of abuse, with the hope that reduction of this arousal will diminish drug reward and thereby facilitate abstinence.
Prolonged stress alters arousal and can produce hypertension, ulcers, immuno-deficiency, sleep disturbances, cognitive dysfunction, and psychiatric disorders (anxiety, depression, and PTSD). Nevertheless, little is known about the functional physiology of the neurons in the paraventricular nucleus of the hypothalamus (PVH) that initiate stress. Our aim is to characterise neurophysiological and pharmacological mechanisms operating on PVH neurons. The initial focus of these studies is on delineation of PVH activity changes during exposure to stressors, as well as their electrophysiological and calcium signalling responses to specific GABA, glutamate, cholinergic, and CRH receptor agonists and antagonists. The ultimate goal is to identify potential targets for pharmacotherapy of stress and related disorders as well as to provide a framework for evaluating drug interactions with stress responses.
During a 24-hour day, humans cycle from different behavioural states spanning the gamut from highly aroused to deep sleep. High levels of arousal are paramount for the focus of attention required for high levels of cognition and complex learning; however, the function(s) of the states of sleep remains a mystery. One approach to elucidation of the function of sleep is to determine the neurobiology underlying the elimination of wakefulness and generation of the states of sleep. Accordingly, work in our laboratory is conducted to elucidate the neuroactive chemicals involved in mediation of sleep and the level of arousal. Moreover, we investigate changes in sleep patterning associated with other arousal disorders, such as depression and stress. In addition, we examine the actions that pharmacologic agents have on activity levels of neurons involved in the generation of arousal states to gain understanding of the mechanisms of action of clinically relevant drugs.
Stress, and especially stress during brain development (i.e. prenatal stress) significantly potentiates the susceptibility to anxiety and depression disorders later in life. It is, however, unclear which structural and functional stress-elicited changes in brain circuitry that subserve development of depression. Our goal is to understand these changes better through anatomical studies, behavioural observations, as well as in vivo and in vitro electrophysiological investigations of signaling in stress-mediating brain pathways in a prenatal stress model of depression. These studies will aid identification of novel target opportunities for treatment of patients suffering from such debilitating stress-related disorders.
Schizophrenia is a disease that has become better understood within the last decade, because of the generation of several clinically relevant animal models and because of findings in these animal models, which translate well into the clinical condition. We are trying to understand the pathophysiology behind a reduced cognitive function in schizophrenia. Our work uses electrophysiological techniques to record neuronal activity in brain slices prepared from two brain regions, the hippocampus and the prefrontal cortex from schizophrenic animals. We have shown that synaptic transmission of GABAergic neurons becomes less effective in schizophrenic animals and we are currently trying to understand the mechanisms behind this observation.
Group/project members
| Name | Title | Phone | |
|---|---|---|---|
| Jefferson Novaes Gomes | External, Ph.d Student | +4535333859 | |
| Kristi Anne Kohlmeier | Associate Professor | ||
| Sunaina Rafi | PhD Student |
Yang Wang, PhD Student E-mail

