Neuromet - Neurometabolism in health and diseases
We study the biochemical and cellular mechanisms underlying energy and amino acid metabolism in the mammalian brain, as well as metabolic brain disorders.
The research in NeuroMet is focused on energy and amino acid metabolism in the mammalian brain. Primary mouse cell culture systems of neurons and astrocytes from cerebral cortex or cerebellum are extensively employed. In addition, genetically modified animals, organotypical hippocampal cultures, acutely isolated brain tissue, isolated mitochondria and cell lines are applied as model systems. An array of 3H, 15N and 13C isotopes is utilized in the mapping of metabolic pathways and their regulation. HPLC and mass spectrometry are key analytical tools combined with biochemical assays, protein biochemistry, molecular biology and fluorescence-based assays and imaging techniques.
Original manuscripts
12. Koivisto H., Leinonen H., Puurula M., Sayed Hafez H., Alquicer Barrera G., Stridh M., Waagepetersen H., Tiainen M., Soininen P., Zilberter Y., Tanila H. (2016) Chronic pyruvate supplementation increases exploratory behavior and improves spatial learning in young and middle-aged mice. Front. Ageing Neurosci. In press
11. Parpura V., Fisher E.S., Lechleiter J.D., Schousboe A., Waagepetersen H.S., Brunet S., Baltan S., Verkhratsky A. (2016) Glutamate and ATP at the Interface Between Signaling and Metabolism in Astroglia: Examples from Pathology. Neurochem. Res. In press
10. Walls A.B., Flynn S.P., West P.J., Müller M.S., Bak L.K., Bulaj G., Schousboe A., White H.S. (2016) The anticonvulsant action of the galanin receptor agonist NAX-5055 involves modulation of both excitatory- and inhibitory neurotransmission. Epilepsy Res. In press
9. Jayakumar A.R., Bak L.K., Rama Rao K.V., Waagepetersen H.S., Schousboe A., Norenberg M.D. (2016) Neuronal Cell Death Induced by Mechanical Percussion Trauma in Cultured Neurons is not Preceded by Alterations in Glucose, Lactate and Glutamine Metabolism. Neurochem. Res. In press
8. Karaca M., Frigerio F., Migrenne S., Martin-Levilain J., Skytt D.M., Pajecka K., Martin-del-Rio R., Gruetter R., Tamarit-Rodriguez J., Waagepetersen H.S., Magnan C., Maechler P. (2015) Lack of GDH-dependent glutamate oxidation in the brain leads to central energy deficiency reshaping peripheral energy distribution. Cell Reports. In press
7. Bakmand T., Troels-Smith A.R., Dimaki M., Nissen J.D., Andersen K.B., Sasso L., Waagepetersen H.S., Gramsbergen J.B., Svendsen W.E. (2015) Fluidic system for long-term in vitro culturing and monitoring of organotypic brain slices. Biomed Microdevices 17(4):71.
6. Nissen J.D., Pajęcka K., Stridh M.H., Skytt D.M., Waagepetersen H.S. (2015) Dysfunctional TCA-cycle metabolism in glutamate dehydrogenase deficient astrocytes. GLIA. In press
5. Lange S.C., Winkler U., Andresen L., Byhrø M., Waagepetersen H.S., Hirrlinger J., Bak L.K. (2015) Dynamic changes in cytosolic ATP levels in cultured glutamatergic neurons during NMDA-induced synaptic activity supported by glucose or lactate. Neurochem. Res. In press
4. Adrover E., Pallares M.E., Baier C.J., Monteleone M.C., Giuliani F.A., Waagepetersen H.S., Brocco M.A., Cabrera R., Sonnewald U., Schousboe A., Antonelli M.C. (2015) Glutamate neurotransmission is affected in prenatally stressed offspring. Neurochem. Int. 88:73-87.
3. Voss C.M., Pajęcka K., Stridh M.H., Nissen J.D., Schousboe A., Waagepetersen H.S. (2015) AMPK activation affects glutamate metabolism in astrocytes. Neurochem. Res. In press
2. Pajęcka K., Nissen J.D., Stridh M.H., Skytt D.M., Schousboe A., Waagepetersen H.S. (2015) Glucose replaces glutamate as energy substrate to fuel glutamate uptake in glutamate dehydrogenase deficient astrocytes. J. Neurosci. Res. 93(7):1093-1100.
1. Müller M.S., Pedersen S.E., Walls A.B., Waagepetersen H.S., Bak L.K. (2015) Isoform-selective regulation of glycogen phosphorylase by energy deprivation and phosphorylation in astrocytes. Glia 63(1):154-162.
Reviews
Satrústegui J. and Bak L.K. (2015) Fluctuations in cytosolic calcium regulate the neuronal malate-aspartate NADH shuttle: implications for neuronal energy metabolism. Neurochem. Res. In press
Hertz L., Chen Y., Waagepetersen H.S. (2015) Effects of ketone bodies in Alzheimer's disease in relation to neural hypometabolism, β-amyloid toxicity and astrocyte function. J. Neurochem. 134(1):7-20.
Books
Waagepetersen H.S., and Schousboe A. (2015) Glial GABA and Glutamate Metabolism. Reference Module in Biomedical Sciences. Elsevier. 08-Aug-15 doi: 10.1016/B978-0-12-801238-3.04618-3.
Schousboe A., Walls A.B., Bak L.K., Waagepetersen H.S. (2015) Astroglia and brain metabolism: focus on energy and neurotransmitter amino acid homeostasis. In: Colloquium Series on Neuroglia in Biology and Medicine: From Physiology to Disease, Parpura & Verkhratsky, eds. Morgan & Claypool Publishers, New Jersy, USA. In press
USA
- Paul Rosenberg, Harvard Uni.
- Kevin Behar, Yale Uni.
- Stephen Moss and Deborah Huyghe, Tufts Uni., Boston
- Brian MacVicar, Uni. of British Columbia, Vancouver
Europe
- Pierre Maechler, Uni. of Geneva
- Ralph Dringen, Uni. of Bremen
- Svante Pääbo, Max Planck Inst. Leipzig
- Ioannis Zaganas, Uni. of Crete
- Mark Cunningham & Felix Chan, Newcastle Uni.
- Heikki Tanila, Uni. Eastern Finland
Denmark
- Poul Hyttel & Kristine Freude, UCPH
- Susanne Keiding, Michael Sørensen, Peter Ott, Hendrik Vilstrup, AU
- Miriam Kolko, UCPH
- Birger Brodin & Hans C. Helms, UCPH
- Winnie E. Svendsen & Maria Dimaki, DTU
Dept. of Drug Design and Pharmacology
- Majid Sheykhzade
- Dan Stærk
- Lasse K. Bak
NeuroMet is currently funded by:
The Danish Medical Research Council
The Lundbeck Foundation
The Hørslev Foundation
The Novo Nordisk Foundation
In addition, NeuroMet has previously received funding from:
The Carlsberg Foundation
The Oticon Foundation
The Aase and Ejnar Danielsen Foundation
Manufacturer Vilhelm Pedersen and Wife Memorial Legacy; this support was granted on recommendation from the Novo Nordisk Foundation
- Anne B. Walls, PostDoc and Associate Professor, until Nov 2018
- Jakob D. Nissen, PostDoc, until Oct 2018
- Felix Chan, visiting PhD student from University of Newcastle, spring 2016
- Michaela Hohnholt, postdoc, 2014 - 2016
- Carla Rubio-Villena, visiting Ph.D. student from Instituto de Biomedicina de Valencia, Spain, April - July 2015.
- Malin H. Stridh, postdoc, 2011-2015.
- Ciara Murray, visiting student, September-December 2013, University College Dublin, Ireland
- Georgia Katsioudi, visiting student, University of Crete, Greece (spring-summer 2013)
- Rebecca Fox, visiting student, September - December 2013, University College Dublin, Ireland
- Karsten K. Madsen, postdoc., 2010 - 2012.
- Ezequiela Adrover, visiting Ph.D. student from Argentina, 2010.
- Joan Borgholm, project student, 2009.
- Renata Leke, visiting Ph.D. student from Federal University of Rio Grande Do Sul, Porto
- Alegre, Brazil, June 2007 - June 2008.
- Marie Werngreen, M.Sc. in Pharmacy, August 2007.
Completed PhD's
Caroline Marie Voss, January 2019. Title: Roles of AMP-activated protein kinase and glutamate dehydrogenase in brain energy metabolism.
Laura Kristine Mcnair, April 2016. Titel: Region Specific Evaluation of Cellular Metabolism in the Brain – Implications of Different Metabolic Mapping Strategies in Combination with NMR or GC-MS
Jakob D. Nissen, April 2015. Title: Role of glutamate dehydrogenase in glutamate metabolism and sustainability of TCA cycle function in astrocytes.
Margit Müller, November 2013. Title: Signaling dynamics in astrocytic glycogenolysis.
Kamilla Pajecka, June 2013. Title: Participation of glutamate dehydrogenase (GDH) in hetero-enzyme complexes: Relation to neurotransmitter homeostasis and brain energy metabolism.
Sherry Dadsetan, June 2013. Title: Mechanisms of metabolism-based ammonia-lowering strategies in the treatment of hepatic encephalopathy.
Dorte M. Skytt, June 2012. Title: Importance of glutamate dehydrogenase, GDH, in astrocyte metabolism for maintenance of glutamate and energy homeostasis.
Linea F. Obel, December 2011. Title: Energy- and amino acid metabolism in astrocytes: Roles of glycogen and Ca2+.
Helle M. Sickmann, May 2009. Title: Glutamatergic neurotransmission under physiological and diabetic conditions - involvement of brain glycogen.
Lasse K. Bak, November 2006. Title: The glutamatergic synapse - aspects of ammonia homeostasis, energy metabolism and glutamine transport.
Completed M. Sc. and B. Sc. Projects
Amaia de Diego Ajenjo, October 2015. Title: Importance of Glutamate Dehydrogenase in glucose and glutamate metabolism and its influence on pyruvate carboxylation in cortical astrocytes from glutamate dehydrogenase knockout mice
Rasmus Kornfelt, April 2015. Titel: A Time Course-Study of [U-13C]Glucose and [1,2-13C]Acetate Metabolism in Acute Cerebellar and Hippocampal Brain Slices
Mathilde Byhrø, April 2015. Title: Role of NMDA receptor activation for oxidative metabolism in cultured neurons.
Caroline M. Voss, April 2015. Title: Activation of AMP-activated protein kinase in astrocytes
Henriette Kihl, November 2013. Title: Coupling of neuronal energy demand and production: Role of mitochondrial cAMP signaling processes.
Nanna Staal, September 2013. Title: Characterization of glucose and glutamate metabolism using [U-13C]glucose og [U-13C]glutamate in cultured hippocampus slices.
Maibrit H. Thoft, September 2013. Title: Characterization of glucose and glutamate metabolism using [U-13C]glucose og [U-13C]glutamate in cultured hippocampus slices.
Camilla W. Nielsen, September 2013. Title: Glutamate dehydrogenase - A study of kinetics and the role of GDH in ATP production.
Marie M. Simonsen, January 2013. Title: Metabolism of branched-chain amino acids following oxygen-glucose deprivation in cortical prisms.
Henrik Ottosen, November 2012. Title: Determination of IC50-values of Hit-8-analogues, GABA-like drugs and isatine derivatives.
Mette M.M. Pedersen, November 2012. Title. A study of the effect of GABAergic enhancing compounds on epileptogenesis in the mouse corneal kindling model.
Stina L. Ehlig-Jensen, November 2012. Title. A study of the effect of GABAergic enhancing compounds on epileptogenesis in the mouse corneal kindling model.
Katja Lassen, October 2012. Title: The impact of GDH activity on energy metabolism in melanoma A375 cells.
Peter Enevoldsen, October 2012. Title: The effect of culturing conditions on primary cultures of astrocytes.
Rikke Søborg, July 2012. Title: Organotypic tissue culture of mice hippocampus; Validation and characterization.
Astrid Hestbæk, May 2012.
Anne Hauge, May 2012. Title: The importance of metabolon formation in brain cell metabolism.
Kasper Pedersen, January 2012. Title: The role of glutamate dehydrogenase 2 in astrocytic energy and amino acid metabolism.
Karen Andersen, August 2011.Title: Adrenergic stimulation of cultured mouse cerebellar astrocytes: A study of Ca2+ signaling and 13C labeling.
Farah S. Jajo, October 2010. Title: Glucose metabolism during increased intracellular calcium concentration in cultured cerebellar neurons.
Sevan A. A. Faek, October 2010. Title: Glucose metabolism during increased intracellular calcium concentration in cultured cerebellar neurons.
Yasar Patruss, September 2010. Title: Role of Glutamate Dehydrogenase in Astrocyte Energy and Amino Acid metabolism.
Bo Juul Aastrup, March 2010. Title: Mitochondrial heterogeneity in the brain.
Sherry Dadsetan, March 2010. Title: Pathophysiology of hepatic encephalopathy.
Trine Krogsbæk Outzen, August 2009. Title: The effect of β-hydroxybutarate on the release of glutamate from cerebellar neurons: Involvement of ATP-sensitive potassium channels.
Malene Anker, November 2008. Title: Involvement of uncoupling in the effect of an elevated ammonia level in the brain
Linea Obel, September 2008. Title: Studying the basic properties of intermolecular glycogenesis and glycogenolysis in the brain
Christina Kjærgaard, March 2008. Title: Metabolism of β-hydroxybutarate in GABAergic neurons.
Johanne Kroon Hansen, September 2007. Title: The metabolism of β-hydroxybutarate in cerebellar neurons.
Marie Werngreen, August 2007.
Stephanie Haut, May 2007. Title: A study of the possible mechanisms underlying the effect of the ketogenic diet.
Charlotte Heimbürger, April 2007. Title: Aspects of glucose and glycogen metabolism in cultured astrocytes.
Søren Døring, November 2006. Title: Characterization of acutely isolated cortical prisms from GAD65 knock-out animals in a perfusion paradigm.
Maja L. Johansen, November 2006. Title: Importance of the branched-chain amino acids during physiological and pathophysiological conditions.
Mette Clausen, August 2006. Title: ß-hydroxybutyrate and glucose metabolism in cultured cerebral cortical neurons.
Anne B. Walls, February 2006. Title: Characterization of a glycogen phosphorylase inhibitor and aspects of glycogen metabolism in the brain.
Helle M. Sickmann, March 2004. Title: Glycogen in the brain - aspects of metabolism and functional importance.
Lasse K. Bak, March 2003. Title: The glutamatergic synapse - aspects of release, uptake and metabolism.
Bachelor's
Sofie E. Pedersen, December 2012. Title: Noradrenalin-induced rise in cytosolic calcium correlates with mobilization of glycogen stores at glycogen phosphorylase B knockdown in vitro
Anna Klawonn, 2008. Title: Importance of GDH in neurons and astrocytes for maintenance of ammonia homeostasis.
Research projects
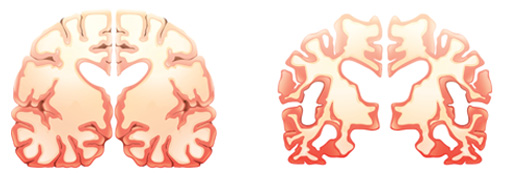
Decline in cerebral glucose metabolism is well established as one of the early pathogenic markers of Alzheimer’s disease (AD) and a potential initiator of neurodegeneration. Impaired insulin signaling, changes in expression and regulation of key enzymes of glucose metabolism and mitochondrial dysfunction all contribute to this hypometabolism.
In NeuroMet we want to characterize the brain hypometabolism in early AD. How does the reduced glucose metabolism develop and how does it affect the disease progression and the vulnerability of neurons and astrocytes?
We are using novel in-vitro models based on neurons and astrocytes either from patient derived tissue or an AD mouse model. By incubating cell cultures with 13C-labeled substrates and subsequently analyzing tissue extracts using nuclear magnetic resonance spectroscopy (NMRS) and mass spectrometry (MS), we get a detailed mapping of the metabolism in the AD models. Furthermore, by using Seahorse Extracellular Flux Analyzer we are able to monitor oxygen consumption to assess the oxidative capacity in the AD models. To evaluate the vulnerability of the cells we apply different AD stressors.
This project will contribute to validation of the AD models used and pave the way for a novel understanding of the coupling between brain energy metabolism and AD. This may lead to a highly needed new approach in AD treatment.
Projects members
Helle S. Waagepetersen
Professor (Head of unit)
helle.waagepetersen@sund.ku.dk
Phone: +45 35 33 64 70
Jakob D. Nissen
Blanca Irene Aldana Garcia
Sofie K. Christensen
Jens V. Andersen
Collaborators
- Heikki Tanila, Uni. Eastern Finland
- Poul Hyttel & Kristine Freude, UCPH
Type 2 diabetes mellitus (T2DM) has reached pandemic proportions as it is estimated that around 7% of the adult world population suffer from T2DM. The effect of T2DM on the human CNS has gained more attention recently as it is becoming evident that T2DM is a risk factor for the development of Alzheimer’s disease. Altered brain energy metabolism is a preclinical symptom of dementia and it has been hypothesized that this could be a mechanistic link between T2DM and Alzheimer’s diseases.
We are studying the effect of T2DM on brain energy metabolism using a common T2DM animal model: the db/db mouse. This transgenic mouse expresses dysfunctional leptin receptors, leading to hyperglycemia and obesity due to overeating.
By investigating brain energy metabolism in acutely isolated brain slices, mitochondria and synaptosomes, we hope to mechanistically unravel some of the mechanisms at work. We apply several different experimental setups and analytical methods, including incubations with stable isotopes (13C) with subsequent analysis by GCMS and HPLC and state-of-the-art mitochondrial assays using the SeaHorse XFe96 flux analyzer.
Our research could help to prevent dementia amplified by T2DM or even reveal possible basal mechanisms to the development of Alzheimer’s disease.
Project members
Helle S. Waagepetersen
Professor (Head of unit)
helle.waagepetersen@sund.ku.dk
Phone: +45 35 33 64 70
Jakob D. Nissen
Sofie K. Christensen
Jens V. Andersen
Lene Arildsen
Collaborators
Majid Sheykhzade, UCPH

How does the brain change in old age? And, can these changes explain the cognitive decline observed in otherwise healthy old individuals? In this project we seek to elucidate alterations in cellular energy metabolism and neurotransmitter homeostasis in the aged mouse brain, hoping to reveal potential targets for counteracting the detrimental effects of brain aging.
The effects of aging on brain functions, especially those involving learning and memory, possess a serious threat to the quality of life in otherwise healthy individuals. The effects have primarily been studied in neurons, and therefore the aim of our current studies are to investigate the effects in astrocytes; a highly abundant and metabolically important glial cell type. In particular we aim to elucidate the effects of aging on cellular energy metabolism and neurotransmitter homeostasis, focusing on metabolic functions unique to the astrocytes.
We target our studies in the hippocampus, which is a brain region highly involved in learning and memory processes and known to be implicated in brain aging. We perform metabolic mapping studies using 13C- or 15N-labelled substrates combined with GC-MS or NMR along with quantitative enzymatic assays and/or HPLC. We employ in vitro as well as in vivo experimental designs in mice, i.e. we incubate acutely isolated hippocampal slices with labelled substrates and perform infusions/injections with labelled substrates in live animals followed by isolation and analysis of the hippocampus.
Understanding the processes and identifying the molecular entities involved in brain aging is crucial to be able to design pharmacological interventions counteracting the detrimental effects of aging on learning and memory functions.
Projects members
Helle S. Waagepetersen
Professor (Head of unit)
helle.waagepetersen@sund.ku.dk
Phone: +45 35 33 64 70
Collaborators
Kevin Behar, Yale Uni.
How does the brain control energy homeostasis- at the same time always being prepared for signaling while not wasting energy?
In the brain energetics project we investigate mechanisms regulating neuronal and astrocytic energy expenditure and production.
We use neurons and astrocytes in culture and isolated mitochondria to monitor respiration, ATP production, calcium signaling and membrane potential changes under different stimuli.
We aim to gain a better understanding of brain energetics to enhance knowledge about mechanisms involved in pathological states such as ischemia after stroke and neurodegenerative diseases.
Projects members
Helle S. Waagepetersen
Professor (Head of unit)
helle.waagepetersen@sund.ku.dk
Phone: +45 35 33 64 70
Anne B. Walls
Caroline M. Voss
Sofie K. Christensen
Collaborators
- Lasse K. Bak
- Brian MacVicar, Uni. of British Columbia, Vancouver
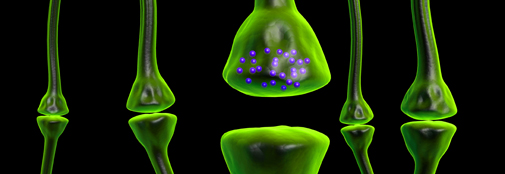
Glutamate is the most abundant excitatory neurotransmitter in the central nervous system. Glutamate is unique as neurotransmitter due to its abundance and its central role in intermediary carbon and nitrogen metabolism. Glutamate metabolism in the brain is compartmentalized and neurons are completely dependent on the ability of astrocytes to synthesize glutamine, the glutamate precursor.
We are eager to complete the puzzle of the role of glutamate and glutamine in brain energetics. To what extent is neuronal activity dependent on the use of amino acids, such as glutamate and glutamine, as energy substrates?
In order to unravel this we use transgenic mouse models in which key proteins are knocked out. We map metabolism employing 13C and 15N labeled substrates, in mice, acute brain slices, in cultured astrocytes and neurons and in synaptosomes. Brain energetics is investigated in isolated mitochondria using the Seahorse technology and on-line ATP assays.
Brain metabolism is challenged in hepatic encephalopathy due to an increased brain ammonia level. Astrocytes are believed to be the major assimilators of ammonia in brain via glutamine synthesis. But how do neurons cope even small amounts of ammonia, we are investigating neuronal nitrogen metabolism in a mouse deficient of their only enzyme assimilating ammonia, namely glutamate dehydrogenase.
Projects members
Helle S. Waagepetersen
Professor (Head of unit)
helle.waagepetersen@sund.ku.dk
Phone: +45 35 33 64 70
Anne B. Walls
Blanca I. Aldana
Caroline M. Voss
Mehdi Khorramis
Collaborators
- Susanne Keiding, Michael Sørensen, Peter Ott, Hendrik Vilstrup, AU
- Pierre Maechler, Uni. of Geneva
- Svante Pääbo, Max Planck Inst. Leipzig
- Ioannis Zaganas, Uni. of Crete
- Paul Rosenberg, Harvard Uni.
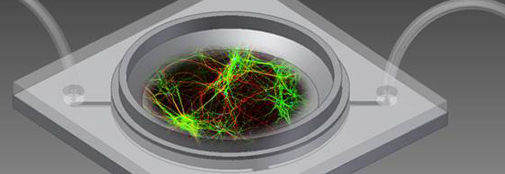
Astrocytes and neurons in culture are used extensively in neuroscience research. However, traditional 2D static cell cultures develop characteristics different from brain cells in the living brain.
In collaboration with NaBIS Nano Bio Integrated Systems, Danish Technical University, we develop novel culture systems in order to improve the in-vivo like characteristics of neurons and astrocytes in culture. We are focusing on 2 different approaches, the development of dynamic superfusion systems and 3D matrix, both to gain biological relevant in vitro environments.
Projects members
Helle S. Waagepetersen
Professor (Head of unit)
helle.waagepetersen@sund.ku.dk
Phone: +45 35 33 64 70
Collaborators
Winnie E. Svendsen & Maria Dimaki, DTU
Group members
| Name | Title | Phone | |
|---|---|---|---|
| Nielsen, Heidi Marie | Laboratory Technician | +4535336333 |